Behind the scenes of ammonia synthesis
Unveiling the catalyst's secrets
Advertisement
Researchers at the Fritz Haber Institute of the Max Planck Society, in collaboration with the Max Planck Institute of Chemical Energy Conversion and Clariant have unveiled new insights into the complex catalyst systems used in industrial ammonia production. By examining the structural evolution of these catalysts, the study highlights the critical role of promoters in enhancing performance and stability.
© FHI
Unveiling the Catalyst's Secrets
The Haber-Bosch process, a cornerstone of industrial ammonia production, has remained largely unchanged for over a century. However, researchers at the Departments of Inorganic Chemistry and Interface Science of the Fritz Haber Institute, the Max Planck Institute for Chemical Energy Conversion, and Clariant have made significant strides in the mechanistic understanding of the highly complex industrial catalyst that drives this process. By using advanced characterization techniques like operando scanning electron microscopy and near-ambient pressure X-ray photoelectron spectroscopy, the team has decoded the complex interactions within multi-promoted ammonia synthesis catalysts.
Prof. Thomas Lunkenbein, the corresponding author, stated, "Our research provides a deeper understanding of the catalyst's inner workings, revealing how promoters and structural transformations contribute to its efficiency and stability. This knowledge is crucial for developing next-generation catalysts that are both more effective and sustainable."
The Critical Role of Activation
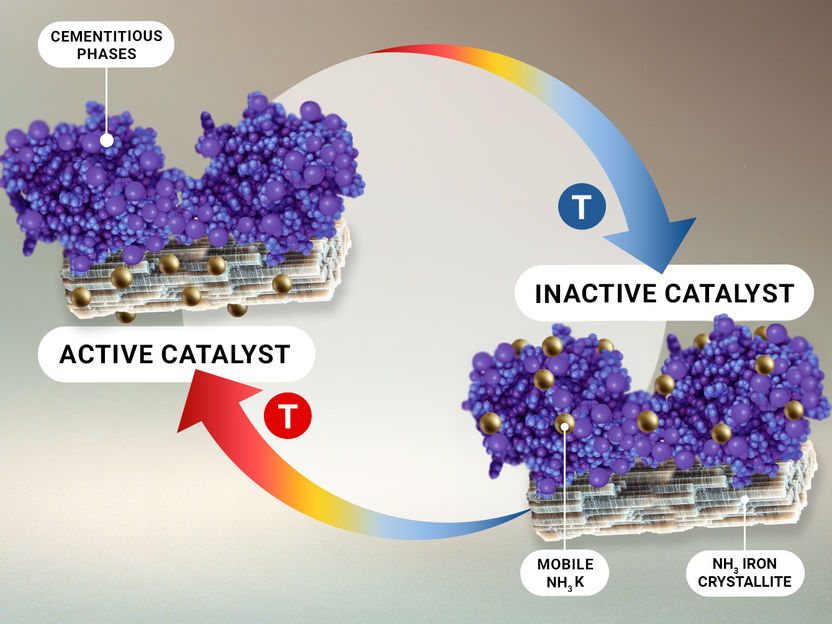
The study reveals that the activation phase is crucial for forming the active catalyst configuration. During this phase, the interplay of various promoter phases facilitates the transformation of the catalyst's structure into a porous entity with a special surface coverage paving the way for its enhanced performance and longevity.
Promoters: The Unsung Heroes
Promoters, including potassium, calcium, and aluminum oxides, are vital in stabilizing the catalyst's structure and boosting its activity. These elements work together to create cement-like phases -an important ingredient for robust and efficient catalyst capable of sustaining ammonia synthesis over extended periods. In addition, ammonia K -a special form of highly dispersed K+ species- was found to be the pacemaker of the catalytic reaction. The research highlights the importance of the catalyst's hierarchical porous structure, which is stabilized by mineral phases. This architecture not only enhances the catalyst's durability but also its resistance to deactivation, ensuring consistent performance in industrial settings.
A Legacy of Innovation
The Fritz Haber Institute of the Max Planck Society has a long history in the field of ammonia synthesis, marked by groundbreaking achievements that have shaped modern industrial chemistry. The institute's namesake, Fritz Haber, was awarded the Nobel Prize in Chemistry in 1918 for his pioneering work on the synthesis of ammonia from its elements, a process that revolutionized agricultural fertilizer production. Decades later, in 2007, Gerhard Ertl, former director of the institute, received the Nobel Prize in Chemistry for his studies of chemical processes on solid surfaces, further advancing the understanding of catalytic reactions, with one of the key examples being the deep mechanistic insight he provided on the ammonia synthesis processes by means of the use of model catalysts. This rich tradition of excellence continues to inspire and drive innovative research at the institute, as evidenced by the latest insights into multi-promoted industrial catalyst systems for ammonia production.
Conclusion
This study sheds light on the intricate dynamics of ammonia synthesis catalysts, offering valuable insights that could pave the way for future innovations in industrial chemistry, including the strong need of considering the dynamic nature of active catalytic surfaces while at work. By understanding the role of promoters and the critical role of the activation process, researchers can develop more efficient and sustainable catalysts for ammonia production. We acknowledge the expertise and input of Prof. Dr. Robert Schlögl, which together with the team of excellent scientists, have led to this important scientific contribution.
Original publication
Luis Sandoval-Díaz, Raoul Blume, Kassiogé Dembélé, Jan Folke, Maxime Boniface, Frank Girgsdies, Adnan Hammud, Zahra Gheisari, Danail Ivanov, René Eckert, Stephan Reitmeier, Andreas Reitzmann, Robert Schlögl, Beatriz Roldan Cuenya, Holger Ruland, Axel Knop-Gericke, Thomas Lunkenbein; "Decoding technical multi-promoted ammonia synthesis catalysts"; Nature Communications, Volume 16, 2025-8-21
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!