Unique chemistry in hydrogen catalysts
Advertisement
Making hydrogen easily and cheaply is a dream goal for clean, sustainable energy. bacteria have been doing exactly that for billions of years, and now chemists at the University of California, Davis, and Stanford University are revealing how they do it, and perhaps opening ways to imitate them.
A study published in the journal Science describes a key step in assembling the hydrogen-generating catalyst.
"It's pretty interesting that bacteria can do this," said David Britt, professor of chemistry at UC Davis and co-author on the paper. "We want to know how nature builds these catalysts — from a chemist's perspective, these are really strange things."
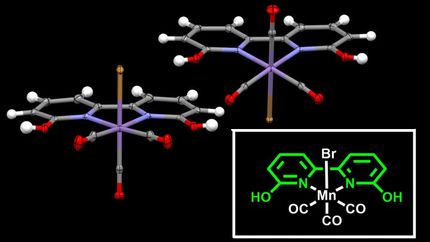
The bacterial catalysts are based on precisely organized clusters of iron and sulfur atoms, with side groups of cyanide and carbon monoxide. Those molecules are highly toxic unless properly controlled, Britt noted.
The cyanide and carbon monoxide groups were known to come from the amino acid tyrosine, Britt said. Jon Kuchenreuther, a postdoctoral researcher in Britt's laboratory, used a technique called electron paramagnetic resonance to study the structure of the intermediate steps.
They found a series of chemical reactions involving a type of highly reactive enzyme called a radical SAM enzyme. The tyrosine is attached to a cluster of four iron atoms and four sulfur atoms, then cut loose leaving the cyanide and carbon monoxide groups behind.
"People think of radicals as dangerous, but this enzyme directs the radical chemistry, along with the production of normally poisonous CO and CN, along safe and productive pathways," Britt said.
Kuchenreuther, Britt and colleagues also used another technique, Fourier Transform Infrared to study how the iron-cyanide-carbon monoxide complex is formed. That work will be published separately.
"Together, these results show how to make this interesting two-cluster enzyme," Britt said. "This is unique, new chemistry."
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.