Desalination gets a graphene boost
New material research making desalination cheaper and more efficient.
Advertisement
When Jeffrey Grossman, a professor at MIT's Department of Materials Science and Engineering (DMSE), began looking into whether new materials might reduce the cost of desalination, he was surprised to find how little research and development money was being applied to the problem.
“A billion people around the world lack regular access to clean water, and that’s expected to more than double in the next 25 years,” Grossman says. “Desalinated water costs five to 10 times more than regular municipal water, yet we’re not investing nearly enough money into research. If we don’t have clean energy we’re in serious trouble, but if we don’t have water we die.”
At the Grossman Group a possible solution may be at hand. Grossman’s lab has demonstrated strong results showing that new filters made from graphene could greatly improve the energy efficiency of desalination plants while potentially reducing other costs as well.
“It’s never been a more exciting time to be a materials scientist,” says Grossman. “When you look at clean tech or water filtration, you find that the energy conversion bottleneck stems from the material. We can now design materials pretty much all the way down to the scale of the atom in almost any way we want, tailoring materials in ways that were previously impossible. There’s a convergence emerging in which we are facing enormously pressing problems that can only be solved by developing new materials.”
Graphene filters: Up to 50 percent less energy
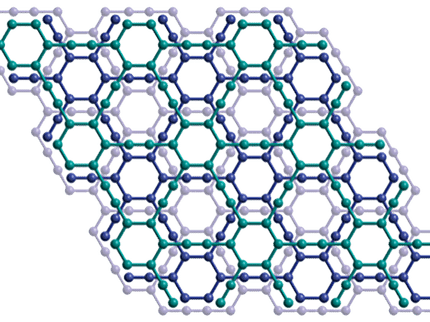
“We have shown that perforated graphene filters can handle the water pressures of desalination plants while offering hundreds of times better permeability,” Grossman explains. “The process of pumping seawater through filters represents about half the operating costs of a desalination plant. With graphene, we could use up to 50 percent less energy.”
Another advantage is that graphene filters don’t become fouled with bio-growth at nearly the rate that occurs with polyamide filters. Desalination plants often run at reduced efficiency due to the need to frequently clean the filters. In addition, the chlorine used to clean the filters reduces the structural integrity of the polyamide, requiring frequent replacement. By comparison, graphene is resistant to the damaging effects of chlorine.
According to Grossman, you could easily replace polyamide filters with graphene filters in existing plants. Like polyamide filters, graphene filters can be mounted on robust polysulfone supports, which have larger holes that sieve out particulates.
Yet, significant challenges remain in bringing down costs. The Grossman Group has made good progress in creating high volumes of graphene at a reasonably low cost. A more serious challenge, however, is cost-effectively poking uniform holes in the graphene in a highly scalable manner.
“A typical plant has tens of thousands of membranes, configured in two-meter long tubes, each of which has 40 square meters of rolled up active membrane,” Grossman says. “We have to match that volume at the same cost, or it’s a nonstarter.”
Making graphene on the cheap
The Grossman Group is using a affordable chemical approach, which produces sufficient quality for creating desalination membranes. “Fortunately, our application doesn’t require the best quality,” says Grossman. “With the chemical technique, we put graphite in a solution, and apply low temperature chemistry to break apart the entire chunk of graphite into sheets. We can get lots of graphene very cheaply and quickly.”
Creating pores that block salt but let water molecules pass is a steeper challenge. The reason desalination is possible in the first place is that when diffused in water, salt ions bond with water molecules, thereby creating a larger entity. But the difference in size compared to a free water molecule is still frustratingly small.
“The challenge is to find the sweet spot of about 0.8 nanometers,” Grossman says. “If your pores are at 1.5 nm, then both the water and salt will pass through. If they’re half a nanometer, then nothing gets through.”
A 0.8 nm hole is “smaller than we’ve ever been able to make in a controllable way with any other material,” Grossman says. “And we need to do this over a very large area very consistently and cheaply.”
The Grossman Group is pursuing three techniques to make nanoporous graphene membranes, all of which use chemical and thermal energy rather than mechanical processes. “If you tried to use lithography, it would take years,” Grossman says. “Our first approach involves making the holes too big, and then carefully filling them in. Another tries to make them exactly the right size, and the third involves starting with a material without holes and then carefully ripping it apart.”
The chemical technique for making graphene actually produces graphene oxide, which is considered undesirable for semiconductors, but is fine for filters. As a result, the researchers were able to avoid the difficult step of removing the oxygen from the graphene oxide. In fact, they found a way to use the oxygen to their advantage.
“By controlling the way the oxygen is bonded to the graphene sheet, we can use chemical and thermal energy to drill the holes with the help of the oxygen,” Grossman says.