How nanoparticles affect flow through porous stuff in surprising ways
Advertisement
Those who have mixed oil and vinegar may have unknowingly observed a strange fluid phenomenon called fingering instability. A type of this phenomenon, called viscous fingering (VF), occurs in porous media where fluids of differing viscosity converge in finger-shaped patterns as a result of growing disturbances at the interface.
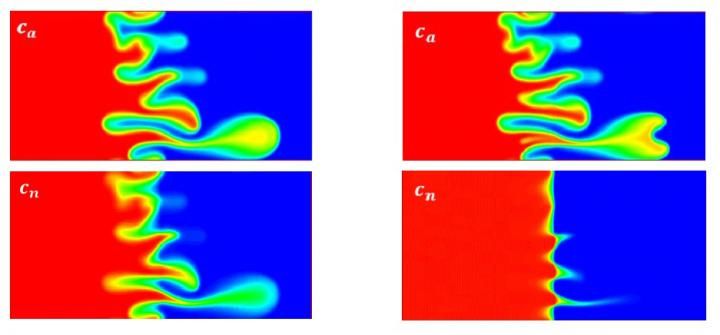
These are countours of c_a and c_n at two nanoparticle deposition rates at t=400
Dastvareh and Jalel Azaiez
Such instabilities are encountered in a wide variety of fields. For applications like the oil recovery process, or contaminant transport in soil, where a fluid is injected to displace oil or contaminants, a uniform fluid front is required to achieve the highest volumetric sweep and effectiveness, making such instabilities undesirable.
On the other hand, in microfluidic devices such as micromixers where inertial effects are negligible, VF is an effective means of enhancing the mixing rate of the fluids. Understanding different aspects of this phenomenon, and the variables that can control things like instabilities and velocity distribution dynamics, can potentially offer options to control and utilize these effects more effectively.
A team of researchers at the University of Calgary has been working on this area for a long time and recently made great strides in understanding the phenomenon.
"My work is part of the puzzle in the evolution in this research area," said Benham Dastvareh, a researcher at the University of Calgary. "My research allows me to combine my interest in mathematics, numerical methods and fundamental research in transport phenomena, and particularly fluid mechanics."
Employing a comprehensive approach, the Calgary researchers incorporated the nonlinear simulation of the growing fingers and also analytical stability analysis of nanofluid displacement in a porous media. By combining the advantages of these methods, they achieved better and more comprehensive understanding of the phenomenon.
Results revealed that nanoparticles cannot make an otherwise stable flow unstable, but they can enhance or attenuate the instability of an originally unstable flow. Increasing either the nanoparticles' deposition rate or their rate of diffusion destabilized the flow. Furthermore, nanoparticle deposition can change an initial monotonically decreasing viscosity distribution -- one that is purely decreasing or unchanging, to a non-monotonic one, and results in the development of vortex dipoles.
"Analyses of vortex structures along with the viscosity distributions allowed us to explain the observed trends and the resulting finger configurations, Dastvareh said. "This work opens a gate for further studies and represents new findings that can be used to control the growing instabilities in the presence of nanofluids for different applications."
This work may also have potential applications for drug delivery, where nanoparticles can't penetrate easily through a porous medium. "It is possible that viscous fingering could be used to open a channel in the human tissue to transfer these nanoparticles for clinical treatment," Dastvareh said.