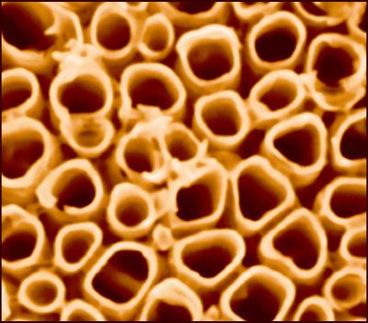
Reactor made of Gold Tubes
Gold nanotubes in polycarbonate films for the investigation of catalytic reactions at gas-liquid phase boundaries
fuel cells require hydrogen. Unfortunately, hydrogen produced by standard processes contains large amounts of carbon monoxide (CO), which has a negative effect on the function of the fuel cell and must be removed. Research has shown that gold nanoparticles on a support with a large surface area are good catalysts for the room-temperature oxidation of CO to CO2. But what is the gold doing in this process-and what is the role of the support? Researchers at the University of Wisconsin have developed a "membrane reactor", which allows them to examine the catalyst without its support.
What is the best way to study a catalyst made of nanoscopic particles in its "pure" state, without a support? The team headed by James A. Dumesic had a clever idea. The researchers took an ultrathin plastic membrane made of polycarbonate containing pores with a diameter of 220 nm. After the surface was specially prepared, gold was deposited onto the membrane. When the precious metal settled onto the walls of the tiny pores, pure gold nanotubes were formed. A subsequent etching process selectively removed the upper layer of the polycarbonate membrane, so that the gold nanotubes protruded from the surface. The researchers stretched this membrane between two chambers, one of which was used to admit gases, the other liquids. Indeed, just like gold nanoparticles, the gold nanotubes catalyzed the reaction of CO and O2 to form CO2.
Systematic examination of the reaction revealed the following: The catalytic activity is increased by the presence of water in the tubes, and is raised still further if the pH level is raised (the solution is made more alkaline). It is clear that hydroxyl groups (OH-), which come to the gold surface from basic materials or the dissociation of water molecules, facilitate the interaction between CO and O2, which seems to result in CO2 and peroxidic intermediates. This theory is supported by the fact that the reaction speeds for supported gold nanoparticles strongly depend on the type of material used for the support. Gold nanoparticles on oxide-containing supports in a damp atmosphere are most active, which fits the theory, since hydroxyl groups are also found under those conditions.
With hydrogen peroxide instead of oxygen as the oxidizing agent, the reaction runs better still, presumably because the bond between the two oxygen atoms in the former is easier to break.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.