Bristol-Myers Squibb Selects Pharmaceutically Relevant Targets For Drug Discovery From Lexicon's LexVision™ Database
Advertisement
Lexicon Genetics Incorporated (Nasdaq: LEXG) announced today that the Bristol-Myers Squibb Company (NYSE:BMY) has selected certain drug targets of potential pharmaceutical relevance contained in LexVision for use in its drug discovery programs.
"Bristol-Myers Squibb’s request for the in vivo mammalian models representing various targets confirms the value that the LexVision database provides for drug discovery, particularly in light of the fact that we are in the early days of this collaboration," said Arthur T. Sands, M.D., Ph.D., President and Chief Executive Officer of Lexicon. "By integrating the full spectrum of in vivo mammalian target validation information into a sophisticated relational database, LexVision provides a consistent and highly-detailed knowledge repository that is focusing drug discovery research on the most pharmaceutically relevant targets."
"We are very happy with the in vivo target validation information in the LexVision database and the value it continues to deliver," said Mark Cockett, Executive Director of Functional Genomics at Bristol-Myers Squibb. "We expect LexVision will continue to add value to our internal drug discovery programs as we move forward in our collaboration with Lexicon."
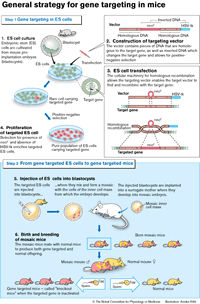
The LexVision program was created to discover the physiologic functions and medical importance of genes that are thought to be potential drug targets. Lexicon analyzes thousands of mouse gene knockouts using an integrated platform of leading edge, medically-relevant tests. This state-of-the art technology platform enables Lexicon to assess the phenotype, or physiological effects, of the knocked-out gene across a variety of parameters relevant to human diseases such as cancer, cardiovascular disease, immune disorders, neurological disease, diabetes and obesity. The information resulting from this analysis is captured in the LexVision database for use by Lexicon and its collaborators for the discovery of genomics-based pharmaceutical products.
Bristol-Myers Squibb became Lexicon’s first collaborator for the LexVision program in September of 2000. In June 2001, Incyte Genomics, Inc. became the second collaborator to join LexVision for its internal drug discovery efforts. Under their respective agreements, Bristol-Myers Squibb and Incyte have access to Lexicon’s LexVision database and OmniBank library for the discovery of small molecule drugs. Both agreements call for in vivo characterization of 250 genes for each year and have maximum terms of five years. Lexicon may receive milestone payments and royalties on any small molecule compounds that are ultimately developed against targets licensed by LexVision subscribers.
As previously announced, Lexicon delivered the third installment of its database to Bristol-Myers Squibb during the second quarter of 2001, triggering a further payment from Bristol-Myers Squibb for its first year of access to LexVision.