Monash team learns from nature to split water
Advertisement
An international team of researchers led by Monash University has used chemicals found in plants to replicate a key process in photosynthesis paving the way to a new approach that uses sunlight to split water into hydrogen and oxygen.
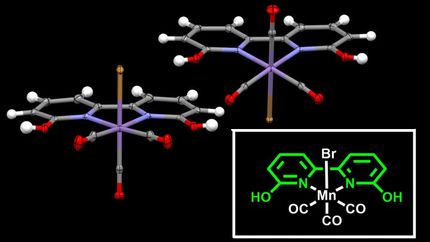
Professor Leone Spiccia, Mr Robin Brimblecombe and Dr Annette Koo from Monash University teamed with Dr Gerhard Swiegers at the CSIRO and Professor Charles Dismukes at Princeton University to develop a system comprising a coating that can be impregnated with a form of manganese, a chemical essential to sustaining photosynthesis in plant life.
"We have copied nature, taking the elements and mechanisms found in plant life that have evolved over 3 billion years and recreated one of those processes in the laboratory," Professor Spiccia said.
"A manganese cluster is central to a plant's ability to use water, carbon dioxide and sunlight to make carbohydrates and oxygen. Man-made mimics of this cluster were developed by Professor Charles Dismukes some time ago, and we've taken it a step further, harnessing the ability of these molecules to convert water into its component elements, oxygen and hydrogen," Professor Spiccia said. "The breakthrough came when we coated a proton conductor, called Nafion, onto an anode to form a polymer membrane just a few micrometres thick, which acts as a host for the manganese clusters."
"Normally insoluble in water, when we bound the catalyst within the pores of the Nafion membrane, it was stabilised against decomposition and, importantly, water could reach the catalyst where it was oxidised on exposure to light."
This process of "oxidizing" water generates protons and electrons, which can be converted into hydrogen gas instead of carbohydrates as in plants.
"Whilst man has been able to split water into hydrogen and oxygen for years, we have been able to do the same thing for the first time using just sunlight, an electrical potential of 1.2 volts and the very chemical that nature has selected for this purpose," Professor Spiccia said.
Testing revealed the catalyst assembly was still active after three days of continuous use, producing oxygen and hydrogen gas in the presence of water, an electrical potential and visible light.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.