50x more stable adsorbent produced
Advertisement
A KAIST research team developed a technology to increase the stability of amine-containing adsorbents by fifty times, moving one step further toward commercializing stable adsorbents that last longer.
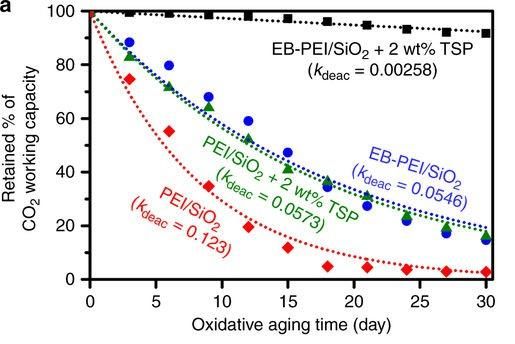
This figure shows carbon dioxide working capacity against oxidative aging time. Performance of the proposed method (black) degrades much more slowly (
KAIST
Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering and his team succeeded in developing amine-containing adsorbents that show high oxidative stability.
The capture of the greenhouse gas carbon dioxide is an active ongoing research field, and some of the latest advancements point to amine-containing adsorbents as an efficient and environment-friendly way to capture carbon dioxide. However, existing amine-containing adsorbents are known to be unstable under oxidation, which chemically breaks down the adsorbent, thereby making it difficult to rely on amine-containing adsorbents for repeated and continued use.
The researchers have discovered that the miniscule amount of iron and copper present in the amine accelerate the oxidative breakdown of the amine-containing adsorbent. Upon this discovery, they proposed the use of a chelator substance, which essentially suppresses the activation of the impurities.
The team demonstrates that the proposed method renders the adsorbent up to 50 times slower in its deactivation rate due to oxidation, compared to conventional polyethyleneimine (PEI) / silica adsorbents. Figure 1 illustrates the superior performance of this oxidation-stable amine-containing adsorbent (shown in black squares), whose carbon dioxide-capturing capacity deteriorates by only a small amount (~8%). Meanwhile, the carbon dioxide-capturing capacity of the PEI/silica adsorbent (shown in red diamonds) degrades dramatically after being exposed to oxidative aging for 30 days.
This stability under oxidation is expected to have brought amine-containing adsorbents one step closer to commercialization. As such, first author Woosung Choi describes the significance of this study as "having brought solid carbon dioxide adsorbents to commercializable standards". In fact, Professor Choi explains that commercialization steps for his team's carbon dioxide adsorbents are already underway. He further set forth his aim to "develop the world's best carbon dioxide capture adsorbent".






























































