Carbon nanotubes show a love/hate relationship with water
Advertisement
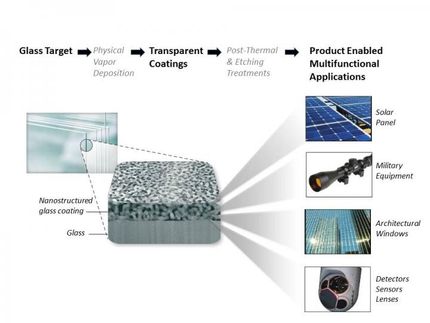
carbon nanotubes (CNTs) are valuable for a wide variety of applications. Made of graphene sheets rolled into tubes 10,000 times smaller than a human hair, CNTs have an exceptional strength-to-mass ratio and excellent thermal and electrical properties. These features make them ideal for a range of applications, including supercapacitors, interconnects, adhesives, particle trapping and structural color.
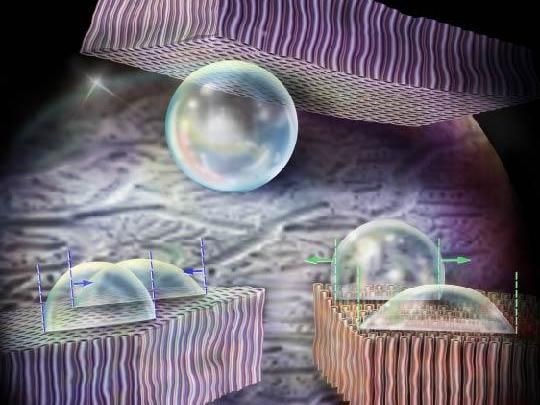
An illustration shows the wetting behavior of carbon nanotubes, which both repel water and hold it in place.
University of Pittsburgh
New research reveals even more potential for CNTs: as a coating, they can both repel and hold water in place, a useful property for applications like printing, spectroscopy, water transport, or harvesting surfaces. When water is dropped on a CNT forest, the CNTs repel the water, and it forms a sphere. However, when flipped over, the drop does not fall to the ground but rather clings to the surface.
"In contrast to superhydrophobic surfaces where droplets roll off easily when tilted, CNTs forests are parahydrophobic, where the droplet is both repelled and attracted to the CNT surface," explains Ziyu Zhou, lead author of the paper and graduate student in the LAMP Lab. "It is a love-hate relationship."
The key to this wetting behavior is the use of CNT forests that are densely, vertically packed on the surface and the inherently hydrophilic CNT surface. The forests are about 100 microns in height and so dense that there are over 100 billion (1011) CNTs in 1 cm2 area. Some amount of water sinks below the carbon nanotubes and clings to the hydrophilic material, while the rest is repelled into a sphere.
This research represents the first observation of parahydrophobicity of CNT forests, where the droplet can roll along the surface but does not fall off when turned upside down. Other surfaces in nature such as peach fuzz or rose petals also exhibit this wetting behavior, which may be used to for liquid transportation, fabrics coating design, membrane selectivity and even wall-climbing robotics.
This wetting behavior could also be used to as a way to construct CNTs into various arrangements.
"Previous research showed CNT forests to be unstable under the application of water, but we show that water droplets are, in fact, stable on these dense CNT forests," explains Paul Leu, PhD, associate professor of industrial engineering at the University of Pittsburgh's Swanson School of Engineering and author on the paper. "This wetting behavior may be used to assemble CNTs into dense vertical arrays, surface stripes, and other unique shapes that could be used for supercapacitors, interconnects, and other applications."