Negatively charged and yet pretty cold
This enables, for example, new investigations of chemical reactions in space
Advertisement
anions, negatively charged ions, are reluctant to be cooled. Physicists led by Matthias Weidemüller from Heidelberg University and Roland Wester from the University of Innsbruck have now developed a method for cooling molecular anions to below 3 Kelvin in a remarkably short time. This enables, for example, new investigations of chemical reactions in space.
Cooling atoms and ions to near absolute zero is routine in many laboratories today. The particles can be very well controlled at these temperatures, and such systems provide an ideal platform for exploring many scientific questions and are the basis for precision clocks or quantum bits. Surprisingly, however, negatively charged ions, known as anions, elude these scientific efforts. They are difficult to cool down. Researchers at Heidelberg University and the University of Innsbruck have now jointly developed a new technique for selectively sorting out the warmest particles from a cloud of molecular ions and thus cooling the remaining molecular ions to below 3 Kelvin.
Evaporative cooling of anions
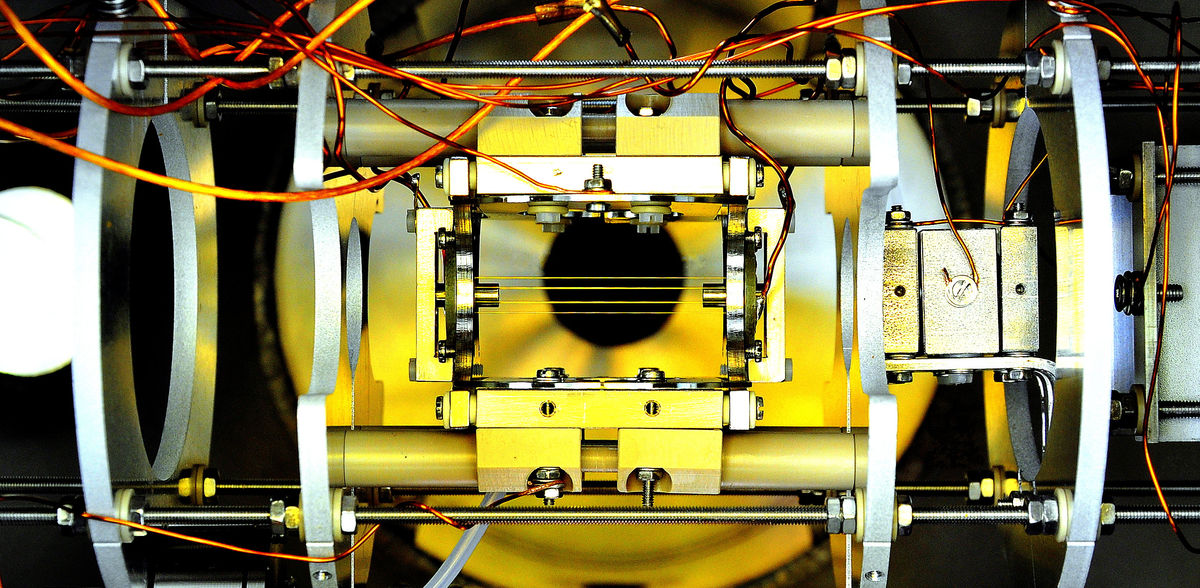
In the experiment, the ions are enclosed in a radio frequency trap and spread along the longitudinal axis of the trap. This allows ions with higher energy to move further away from the center of the trap. "We exploit this to selectively remove these ions from the trap," says Eric Endres of the Department of Ion Physics and Applied Physics. "Using a focused laser beam positioned at the edge of the ion cloud, we neutralize the warmer ions. The photons from the laser thereby detach an electron from the anion, creating a neutral molecule that drops out of the trap." After the higher-energy ions evaporate, the remaining ions cool to a lower temperature. "By slowly moving the laser light towards the trap center, the highest energy anions are evaporated one by one, leading to a temperature of 2.2 Kelvin in less than four seconds," explains Saba Hassan from the Institute for Physics of Heidelberg University.
Previously used techniques allow cooling of anions down to 3 Kelvin. "With our further developed method, this barrier can now in principle be broken for any kind of negatively charged molecule, allowing many new investigations into the fundamentals of nature or, for example, of chemical reactions in space," research group leaders Matthias Weidemüller and Roland Wester are delighted to say.