Clue for efficient usage of low-cost nickel catalysts
Advertisement
A group of researchers at Osaka University developed a method of the consecutive formation of bonds of two butadiene, alkyl groups, and benzene rings by using a cheap nickel catalyst. Using this technique, it has become possible to synthesize high-value terminal olefin by using cheap butadiene.
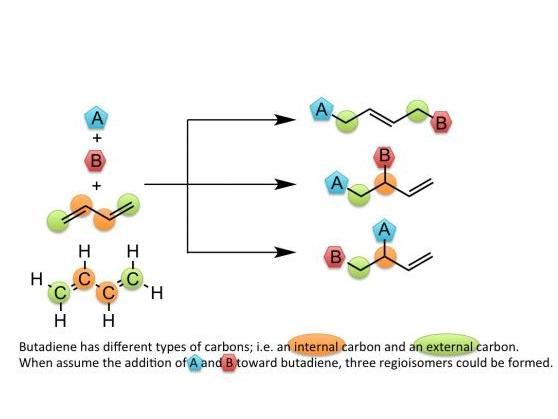
This image shows possible products in the multicomponent reaction of butadiene.
Osaka University
Multicomponent reactions are methods which are superior in economy and efficiency to methods of bonding molecules by repeating reactions, but it was necessary to control the number of molecules to be bonded and locations of the bonds, so their applications were limited.
Nobuaki Kambe, Professor, and Takanori Iwasaki, Assistant Professor, at the Graduate School of Engineering, Osaka University developed a synthetic method by constructing carbon frameworks of 8 carbons through the formation of a bond of two butadiene molecules by using a cheap nickel catalyst and introducing an alkyl group and a benzene ring to an internal and terminal carbons of 1,6-octadiene, respectively. Using this technique, it has become possible to synthesize high-value terminal olefin by using cheap butadiene.
Using the same butadiene used in a wide range of fields as materials for synthetic rubber, such as tires and rubber hoses, and as materials for important industrial chemical compounds, such as butanediol and chloroprene. Recently, this group succeeded to develop the synthetic method of branched terminal olefins from butadiene and alkyl halides through selective introduction of alkyl group into the internal carbon of butadiene by the aid of Cu catalyst. This group's achievements will lead to the development of methods for synthesizing various organic materials from butadiene by using different catalysts.
Furthermore, it is possible to synthesize butadiene from ethanol. This group's achievement demonstrates the possibility of changing high-value chemical compounds such as bioethanol, which has been gathering attention in recent years, into different chemical compounds by using catalysts differently.































































