Electroplating delivers high-energy, high-power batteries
Advertisement
The process that makes gold-plated jewelry or chrome car accents is now making powerful lithium-ion batteries.

The electroplating method could enable flexible, three-dimensional battery designs. This plated aluminum foil rolled up without cracking.
Image courtesy of Hailong Ning and Jerome Davis III, Xerion Advanced Battery Corp.
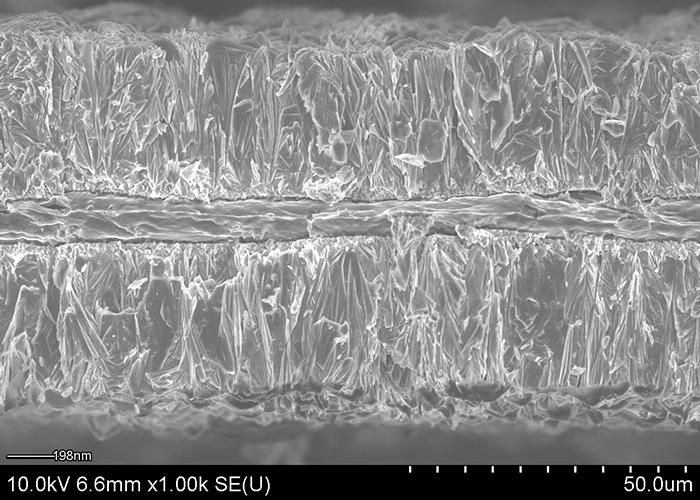
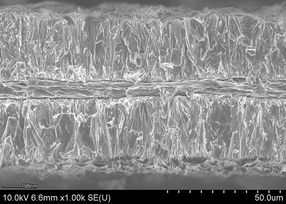
An electron micrograph cross-section shows aluminum foil plated with lithium cobalt oxide, a common material in lithium-ion batteries.
Image courtesy of Hailong Ning and Jerome Davis III, Xerion Advanced Battery Corp.

Researchers at the University of Illinois, Xerion Advanced Battery Corporation and Nanjing University in China developed a method for electroplating lithium-ion battery cathodes, yielding high-quality, high-performance battery materials that could also open the door to flexible and solid-state batteries.
"This is an entirely new approach to manufacturing battery cathodes, which resulted in batteries with previously unobtainable forms and functionalities," said Paul V. Braun, a professor of materials science and engineering and director of the Frederick Seitz Materials Research Lab at Illinois.
Traditional lithium-ion battery cathodes use lithium-containing powders formed at high temperatures. The powder is mixed with gluelike binders and other additives into a slurry, which is spread on a thin sheet of aluminum foil and dried. The slurry layer needs to be thin, so the batteries are limited in how much energy they can store. The glue also limits performance.
"The glue is not active. It doesn't contribute anything to the battery, and it gets in the way of electricity flowing in the battery," said co-author Hailong Ning, the director of research and development at Xerion Advanced Battery Corporation in Champaign, a startup company co-founded by Braun. "You have all this inactive material taking up space inside the battery, while the whole world is trying to get more energy and power from the battery."
The researchers bypassed the powder and glue process altogether by directly electroplating the lithium materials onto the aluminum foil.
Since the electroplated cathode doesn't have any glue taking up space, it packs in 30 percent more energy than a conventional cathode, according to the paper. It can charge and discharge faster as well, since the current can pass directly through it and not have to navigate around the inactive glue or through the slurry's porous structure. It also has the advantage of being more stable.
Additionally, the electroplating process creates pure cathode materials, even from impure starting ingredients. This means that manufacturers can use materials lower in cost and quality and the end product will still be high in performance, eliminating the need to start with expensive materials already brought up to battery grade, Braun said.
"This method opens the door to flexible and three-dimensional battery cathodes, since electroplating involves dipping the substrate in a liquid bath to coat it," said co-author Huigang Zhang, a former senior scientist at Xerion who is now a professor at Nanjing University.
The researchers demonstrated the technique on carbon foam, a lightweight, inexpensive material, making cathodes that were much thicker than conventional slurries. They also demonstrated it on foils and surfaces with different textures, shapes and flexibility.
"These designs are impossible to achieve by conventional processes," Braun said. "But what's really important is that it's a high-performance material and that it's nearly solid. By using a solid electrode rather than a porous one, you can store more energy in a given volume. At the end of the day, people want batteries to store a lot of energy."
Original publication
Zhang, Huigang and Ning, Hailong and Busbee, John and Shen, Zihan and Kiggins, Chadd and Hua, Yuyan and Eaves, Janna and Davis, Jerome and Shi, Tan and Shao, Yu-Tsun and Zuo, Jian-Min and Hong, Xuhao and Chan, Yanbin and Wang, Shuangbao and Wang, Peng and Sun, Pengcheng and Xu, Sheng and Liu, Jinyun and Braun, Paul V.; "Electroplating lithium transition metal oxides"; Science Advances; 2017
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.