Plutonium Decontamination Agent Characterized at Berkeley Advanced Light Source
Advertisement
In an on-going effort to design and synthesize chemical substances that can safely and effectively remove plutonium and other radioactive materials from the human body or from the environment, scientists at the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have made an important advance. Using the exceptionally bright and intense x-ray beams of Berkeley Lab's Advanced Light Source (ALS), they have determined the crystal structure of a molecular complex that has shown promise as a sequestering agent for plutonium and other members of the actinide family of elements.
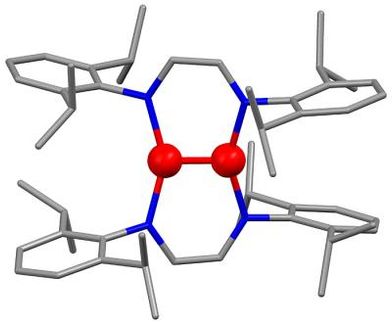
"This is the first plutonium complex that has been characterized using single crystal x-ray diffraction with a synchrotron radiation source like the ALS," says Anne Gorden, the Glenn T. Seaborg Center Postdoctoral Fellow with Berkeley Lab's Chemical Sciences Division (CSD), and one of the co-authors of a paper on this work which appears in an upcoming issue of Chemistry, a European Journal.
"The need for agents that will bind with plutonium and other actinides, and immobilize them for removal from living tissue, or allow them to be sequestered from the environment is more urgent than ever," says Gorden. "However, scientists first need to develop a better understanding of basic actinide chemistry."
"Because it is very difficult to crystallize plutonium and other actinide complexes, being able to do the diffraction work on a synchrotron like the ALS is a huge advantage," says Gorden. "We can work with crystals as small as 15 microns to a side, and collect data in about 45 minutes that would take 10 hours or more using a conventional x-ray source."
The plutonium crystals that Gorden and her colleagues investigated were ether-bridged hydroxypyridonate ligands nicknamed HOPO, which are based on the chelating unit found in siderophores, small molecules that are secreted by bacteria to extract and solubilize iron. For the past two decades, collaborator Ken Raymond has been leading investigations into the development of HOPO and other complexing agents that would encapsulate plutonium into tightly bound cage-like complexes.
In tests on mice, HOPO complexes have stood out as being far more effective at removing plutonium and other actinides from the body than the current approved treatments. Also, HOPO complexes could be administered orally via a pill, and experiments have demonstrated that it may even remove plutonium that has been deposited in bone.
Cerium, a member of the non-radioactive lanthanide family of elements, has been used as a safe stand-in for plutonium in structural studies. Although the two elements have different chemistry, the Ce(IV) and Pu(IV) ions have a similar size. The Berkeley scientists plan to continue their studies with lanthanides and actinides to determine if the differences in these complexes are due to solvent effects or the interactions between the ligands and these heavy metals.
"Our work with the plutonium HOPO complex indicates that a library of actual actinide structures will be necessary for developing effective actinide decorporation or sequestering agents," Gorden says.