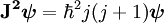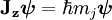To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Azimuthal quantum numberThe Azimuthal quantum number (or orbital angular momentum quantum number) symbolized as l (lower-case L) is a quantum number for an atomic orbital which determines its orbital angular momentum. The azimuthal quantum number is the second of a set of quantum numbers (the principal quantum number, following spectroscopic notation, the azimuthal quantum number, the magnetic quantum number, and the spin quantum number) which describe the unique quantum state of an electron and is designated by the letter l. Product highlight
DerivationThere is a set of quantum numbers associated with the energy states of the electrons of an atom. The four quantum numbers n, l, ml, and ms specify the complete and unique quantum state of a single electron in an atom called its wavefunction or orbital. The wavefunction of the Schrödinger wave equation reduces to the three equations that when solved lead to the first three quantum numbers. Therefore, the equations for the first three quantum numbers are all interrelated. The azimuthal quantum number arose in the solution of the polar part of the wave equation as shown below. An atomic electron's angular momentum, L, which is related to its quantum number where The energy of any wave is the frequency multiplied by Planck's constant. This causes the wave to display particle-like packets of energy called quanta. To show each of the quantum numbers in the quantum state, the formulae for each quantum number include Planck's reduced constant which only allows particular or discrete or quantized energy levels. This behavior manifests itself as the "shape" of the orbital.
Electron shells have distinctive shapes denoted by letters. In the illustration, the letters s, p, and d describe the shape of the atomic orbital. Their wavefunctions take the form of spherical harmonics, and so are described by Legendre polynomials. The various orbitals relating to different values of l are sometimes called sub-shells, and (mainly for historical reasons) are referred to by letters, as follows:
A mnemonic for the order of the "shells" is some poor dumb fool. Another mnemonic for the order of the "shells" is silly professors dance funny. The letters after the F subshell just follow F in alphabetical order. Each of the different angular momentum states can take 2(2l+1) electrons. This is because the third quantum number ml (which can be thought of loosely as the quantised projection of the angular momentum vector on the z-axis) runs from −l to l in integer units, and so there are 2l+1 possible states. Each distinct nlml orbital can be occupied by two electrons with opposing spins (given by the quantum number ms), giving 2(2l+1) electrons overall. Orbitals with higher l than given in the table are perfectly permissible, but these values cover all atoms so far discovered. For a given value of the principal quantum number, n, the possible values of l range from 0 to n−1; therefore, the n=1 shell only possesses an s subshell and can only take 2 electrons, the n=2 shell possesses an s and a p subshell and can take 8 electrons overall, the n=3 shell possesses s, p and d subshells and has a maximum of 18 electrons, and so on (generally speaking, the maximum number of electrons in the nth energy level is 2n2). The angular momentum quantum number, l, governs the number of planar nodes going through the nucleus. A planar node can be described in an electromagnetic wave as the midpoint between crest and trough, which has zero magnitude. In an s orbital, no nodes go through the nucleus, therefore the corresponding azimuthal quantum number l takes the value of zero. In a p orbital, one node traverses the nucleus and therefore l has the value 1. Depending on the value of n, the principal quantum number, there is an angular momentum quantum number l and the following series. The wavelengths listed are for a hydrogen atom:
Addition of quantized angular momenta
Given a quantized total angular momentum the quantum number j associated with its magnitude can range from | l1 − l2 | to l1 + l2 in integer steps where l1 and l2 are quantum numbers corresponding to the magnitudes of the individual angular momenta. Total angular momentum of an electron in the atomDue to the spin-orbit interaction in the atom, the orbital angular momentum no longer commutes with the Hamiltonian, nor does the spin. These therefore change over time. However the total angular momentum J does commute with the Hamiltonian and so is constant. J is defined through L being the orbital angular momentum and S the spin. The total angular momentum satisfies the same commutation relations as angular momentum, namely from which follows where Ji,j stand for Jx, Jy and Jz. The quantum numbers describing the system, which are constant over time, are now j and mj, defined through the action of J on the wavefunction ψ So that j is related to the norm of the total angular momentum and mj to its projection along a specified axis. As with any angular momentum in quantum mechanics, the projection of J along other axes cannot be co-defined with Jz, because they do not commute. Relation between new and old quantum numbers
j and mj, together with the parity of the quantum state, replace the three quantum numbers l, ml and ms (the projection of the spin along the specified axis). The former quantum numbers can be related to the latter. Furthermore, the eigenvectors of j, mj and parity, which are also eigenvectors of the Hamiltonian, are linear combinations of the eigenvectors of l, ml and ms. List of angular momentum quantum numbers
HistoryThe azimuthal quantum number was carried over from the Bohr model of the atom. The Bohr model was derived from spectroscopic analysis of the atom in combination with the Rutherford atomic model. The lowest quantum level was found to have an angular momentum of zero. To simplify the mathematics, orbits were considered as oscillating charges in one dimension and so described as "pendulum" orbits. In three-dimensions the orbit becomes spherical without any nodes crossing the nucleus, similar to a jump rope that oscillates in one large circle. See also
|
|||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Azimuthal_quantum_number". A list of authors is available in Wikipedia. |





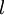 is described by the following equation:
is described by the following equation:
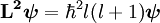
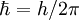 is the reduced Planck's constant, also called Dirac's constant,
is the reduced Planck's constant, also called Dirac's constant, 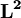 is the orbital angular momentum operator and
is the orbital angular momentum operator and 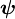 is the wavefunction of the electron. While many introductory text books on quantum mechanics will refer to L by itself, L has no real meaning except in its use as the angular momentum operator. When referring to angular momentum, it is best to simply use the quantum number
is the wavefunction of the electron. While many introductory text books on quantum mechanics will refer to L by itself, L has no real meaning except in its use as the angular momentum operator. When referring to angular momentum, it is best to simply use the quantum number 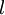 .
.
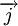 which is the sum of two individual quantized angular momenta
which is the sum of two individual quantized angular momenta 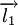 and
and 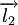 ,
,
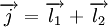
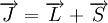
![[J_i, J_j ] = i \hbar \epsilon_{ijk} J_k](images/math/9/c/8/9c806fccd8d3a97bd8f4376bc9d41efa.png)
![\left[J_i, J^2 \right] = 0](images/math/7/f/0/7f0a85c80dc802597f9d3fa1b0c3b22b.png)