To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Boltzmann distribution
In physics, the Boltzmann distribution predicts the distribution function for the fractional number of particles Ni / N occupying a set of states i which each respectively possess energy Ei: Product highlightwhere kB is the Boltzmann constant, T is temperature (assumed to be a sharply well-defined quantity), gi is the degeneracy, or number of states having energy Ei, N is the total number of particles: and Z(T) is called the partition function, which can be seen to be equal to Alternatively, for a single system at a well-defined temperature, it gives the probability that the system is in the specified state. The Boltzmann distribution applies only to particles at a high enough temperature and low enough density that quantum effects can be ignored, and the particles are obeying Maxwell–Boltzmann statistics. (See that article for a derivation of the Boltzmann distribution.) The Boltzmann distribution is often expressed in terms of β = 1/kT where β is referred to as thermodynamic beta. The term exp(−βEi) or exp(−Ei/kT), which gives the (unnormalised) relative probability of a state, is called the Boltzmann factor and appears often in the study of physics and chemistry. When the energy is simply the kinetic energy of the particle then the distribution correctly gives the Maxwell–Boltzmann distribution of gas molecule speeds, previously predicted by Maxwell in 1859. The Boltzmann distribution is, however, much more general. For example, it also predicts the variation of the particle density in a gravitational field with height, if In some cases, a continuum approximation can be used. If there are g(E) dE states with energy E to E + dE, then the Boltzmann distribution predicts a probability distribution for the energy: Then g(E) is called the density of states if the energy spectrum is continuous. Classical particles with this energy distribution are said to obey Maxwell–Boltzmann statistics. In the classical limit, i.e. at large values of E/kT or at small density of states—when wave functions of particles practically do not overlap, both the Bose–Einstein or Fermi–Dirac distribution become the Boltzmann distribution. DerivationSee Maxwell–Boltzmann statistics.
Categories: Particle statistics | Statistical mechanics |
|||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Boltzmann_distribution". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||


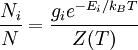



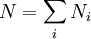
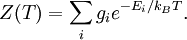
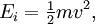
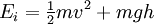 . In fact the distribution applies whenever quantum considerations can be ignored.
. In fact the distribution applies whenever quantum considerations can be ignored.