Green light for a new generation of dynamic materials
Advertisement
Developing synthetic materials that are as dynamic as those found in nature, with reversibly changing properties and which could be used in manufacturing, recycling and other applications, is a strong focus for scientists.
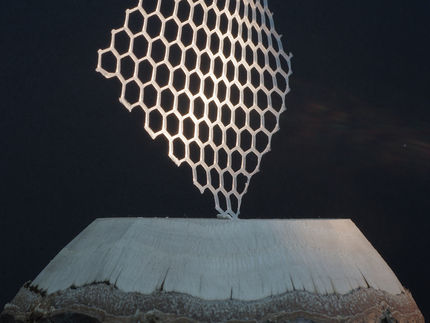
Researchers at QUT, UGent and KIT have pioneered a novel TAD/naphthalene-based light-stabilised dynamic material that is stable under visible green light and becomes fluid over time in darkness.
QUT/UGent/KIT
In a world-first, researchers from Queensland University of Technology (QUT), Ghent University (UGent) and Karlsruhe Institute of Technology (KIT) have pioneered a novel, dynamic, reprogrammable material - by using green LED light and, remarkably, darkness as the switches to change the material's polymer structure, and using only two inexpensive chemical compounds. One of these compounds, naphthalene, is well known as an ingredient in moth repellents.
The new dynamic material could potentially be used as a 3D printing ink to print temporary, easy-to-remove support scaffolds. This would overcome one of the current limitations of the 3D process to print free-hanging structures.
The research is part of an ongoing international collaboration between QUT macromolecular chemist and Australian Research Council Laureate Fellow Professor Christopher Barner-Kowollik, Dr Hannes Houck, who recently completed his PhD across QUT, UGent and KIT, UGent Professor Filip Du Prez, and KIT's Dr Eva Blasco.
Key points:
- The new material was formed with naphthalenes and the coupling molecules triazolinediones (TADs)
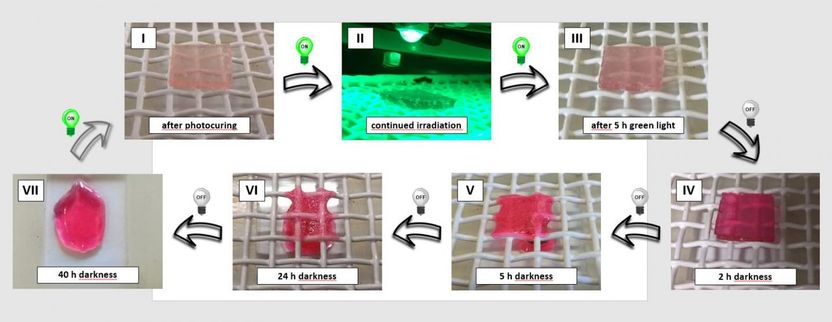
- As long as green LED light shone on the material it remained stable and strong
- Once the light was off and the material was kept in darkness, the chemical bonds of the network structure broke up and the material became soft and liquefied
- The hard-to-soft process could be repeated with the flick of the switch, and the light could be dimmed to modulate the mechanical properties of the material
- Follow on research is looking at other chemical combinations that can achieve the same result
Professor Barner-Kowollik, from QUT's Science and Engineering Faculty, said what makes the discovery unique is that light is used as the trigger to stabilise, rather than destroy, chemical bonds - so the researchers have coined a new term, light-stabilised dynamic materials (LSDMs).
"We are hoping to introduce LSDMs as a whole new class of materials," said Dr Houck. "We debated whether to patent the new material, but decided not to wait and to publish the findings to advance knowledge and understanding of the processes involved."
The researchers said what they have achieved is the opposite of what is usually done in chemistry and "many people didn't think it could be done".
"Typically, you use different wavelengths of light or additional heat or harsh chemicals to break up the polymer molecule chains that form a network structure," they said.
"However, in this case, we used green LED light to stabilise the network. The trigger to break up the network, make it collapse and flow away is actually the mildest one of all: darkness. Switch the light back on and the material re-hardens and retains its strength and stability.
"This is what you call an out-of-equilibrium chemical system. The constant energy of the green light keeps the chemical system in this bonded form, pushing it out of its equilibrium. Take away the light, and the system goes back to its relaxed, lowest energy state."
Professor Barner-Kowollik said the researchers had already been contacted by 3D printing technology companies interested in application of the research.
3D printing is used in the aerospace and automotive industries to make intricate parts and detailed prototypes.
However, 3D printing complex designs with overhangs or bridges is difficult or off limits because the 3D process involves printing layer upon layer, and there is no direct support for layers in sharply angled structures.
"What you need to 3D print something like a bridge is a support scaffold, a second ink that provides that scaffold during printing of the design, but which you can later remove when it is no longer needed," he said.
"With a light-stabilised dynamic ink used as a scaffold you could 3D print under light, then switch the light off to let the scaffold ink flow away."
Professor Du Prez and Professor Barner-Kowollik said another potential application for LSDMs was as a cell biology study tool, with biologists using it as a cell surface support they could alter by light modulation without damaging the cells.