Visible light-photocatalytic water-splitting for hydrogenation of aryl chlorides
Sustainable H-donors instead of flammable hydrogen gas
Advertisement
Activation of inert C-Cl bonds has attracted significant interest due to the inherent fundamental challenge. Conventional dechlorination generally use flammable hydrogen gas (H2), metal hydrides, or organic donors (amines, alcohols, and Hantzsch ester) as hydrogenation reagents.
Controllable Hydrogenation/Deuteration of Aryl Chlorides based on Photocatalytic Water-Splitting Strategy
©Science China Press
Despite high hydrogenation efficiency, these processes have significant drawbacks because of the risks involved while using a flammable gas at high pressure, or needing complex infrastructure to handle air and moisture-sensitive metal hydride reducing agents or expensive organic hydrogen donors. To this end, it is appealing to develop a sustainable process for the hydrogenation of chlorides using sustainable and readily available hydrogen-donors, whereas water is the most ideal candidate.
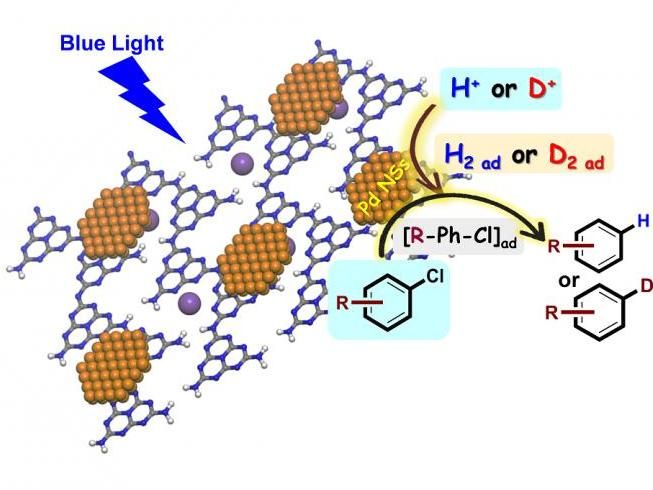
Recently, Chenliang Su's research group from Shenzhen University, China, proposed a photocatalytic water splitting technology to in-situ "bubble" H2 for controllable hydrogenation of aryl chlorides, whereas the water system is utilized as safe and sustainable H-donors instead of flammable H2.
Using isotopically labelled water (D2O), the strategy has been successfully applied to install deuterium at C-Cl of chlorides under mild conditions with high deuterium incorporation and good functional group tolerance for the first time, providing an attractive way for high incorporation of deuterium in drug molecules to improve their pharmacokinetic and toxicity profiles. "Pd nanosheets (Pd NSs) decorated crystallined polymeric carbon nitride (CPCN) are used as the bifunctional photocatalyst, whereas Pd NSs not only serve as cocatalyst of CPCN to generate and stabilize H (D)-species but also play a significant role in sequential activation and hydrogenation/deuteration of C-Cl bonds.", Prof. Su said.





























































