Chemist developed a green catalyst for pharmaceutical and industrial chemistry
Catalyst based on a substance that comes from plant waste
Advertisement
Many production facilities (e.g. plastic manufacturers, pharma companies, and others) use nanocatalysts that contain palladium--an expensive component that is not sustainably produced. A chemist from RUDN University found a way to reduce palladium consumption and to make its manufacture more eco-friendly. He developed a catalyst based on a substance that comes from plant waste. Using his invention, manufacturers could cut palladium consumption in half. Moreover, new catalysts can be reused multiple times without any decrease in efficiency.
Many production facilities (e.g. plastic manufacturers, pharma companies, and others) use nanocatalysts that contain palladium--an expensive component that is not sustainably produced. A chemist from RUDN University found a way to reduce palladium consumption and to make its manufacture more eco-friendly. He developed a catalyst based on a substance that comes from plant waste. Using his invention, manufacturers could cut palladium consumption in half. Moreover, new catalysts can be reused multiple times without any decrease in efficiency.
RUDN University
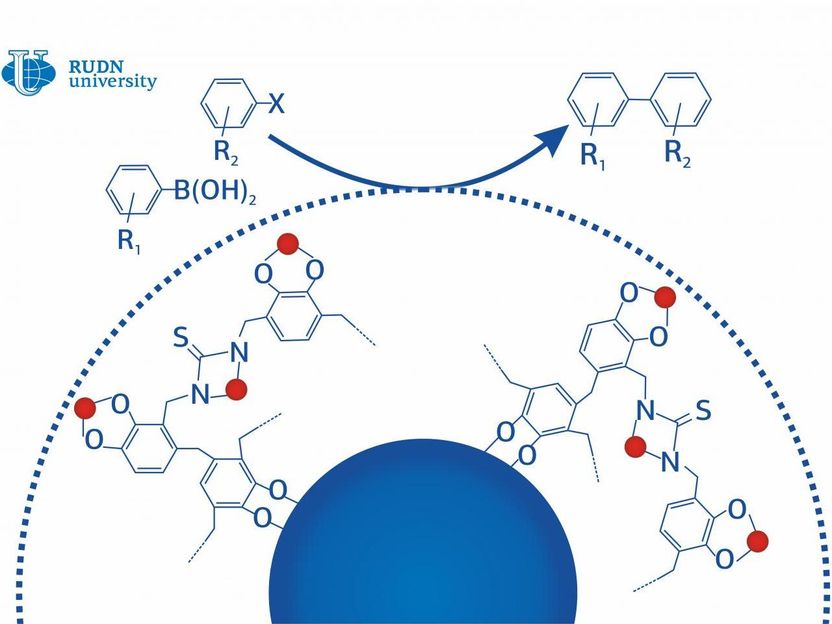
Cross-coupling is a type of reaction that involves combining carbon atoms from different organic molecules. Cross-coupling reactions are most widely spread ones in industrial chemistry. They are used to synthesize plastics, medicinal drugs, and other compounds and account for 17% of all reactions in medical chemistry only. The main component of cross-coupling is palladium nanoparticles. Palladium is one of the rarest elements on Earth, which makes it a very expensive catalyst. Moreover, it is mainly produced at mining facilities that pose a considerable threat to the environment. A chemist from RUDN University suggested solving all these issues with one new approach.
The consumption of palladium in cross-coupling reactions increases because the particles of palladium-containing catalysts tend to bind together. There are two ways to stop this. One could modify the chemical properties of the particles to weaken the reaction between their surfaces when they come in contact. Alternatively, the metal could be held in place physically with a framework or a grid. The chemist from RUDN University chose the second method and locked metal particles in their respective places using a multilayer shell with a magnetic core.
The core of the new nanocatalyst consists of iron oxide with high magnetic properties. The coating is made of a catechol-based polymer. Catechol is a substance that is found in plant cell walls and is produced from plant waste. Both these layers are ancillary and have no catalytic activity. The catalytic properties of the compound come from palladium nanoparticles that are incorporated into the second layer. The polymer fixes the particles in place and prevents them from binding together.
The new catalyst structure requires twice as little palladium as the old one: 1.5% of the total nanoparticle weight as opposed to 3-6%. Moreover, after a couple of production cycles, the core of the nanocomposite material can be cleaned up and reused. This method is not only good for the environment but also economically feasible, as it will make the production of medicinal drugs, plastics, and other products cheaper.
"Today chemists are especially interested in green catalysts. Our nanocatalysts contain a product of plant waste recycling and at the same time efficiently work in cross-coupling reactions. Therefore, not only are they able to reduce palladium consumption and make the production process cheaper, but also are beneficial for the environment. Moreover, we managed to showcase the universal nature of polymers based on plant catechols. The same approach can be used when working with other metals including platinum, silver, or gold, or with catalysts of other organic reactions," said Rafael Luque, PhD, Head of the Molecular Design and Synthesis of Innovative Compounds for Medicine Science Center at RUDN University.