As hard as a diamond and as deformable as metal
Scientists develop new material for tomorrow's technology
Advertisement
Smartphones with large glass housings and displays are impressive, but they are also very prone to get cracked and scratched. To prevent these kinds of damages, a material combining the hardness of diamond and the deformability of metals would be ideal – and is indeed considered the holy grail of structural materials. Professor Gerold Schneider of the Hamburg University of Technology and other Hamburg materials researchers, together with colleagues in Berkeley, California, have now developed a hybrid material, a so-called supercrystal, that comes closer to achieving this goal.
TU Hamburg
Such a material has the potential to make technology in areas such as electronics, photonics and energy storage more robust and cost-effective.
Deformable material made of nanoparticles
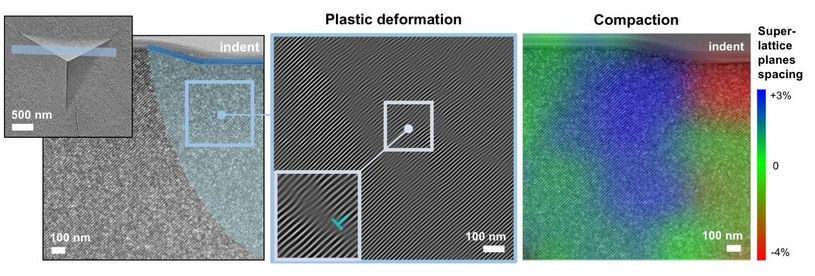
In collaboration with colleagues from the Helmholtz-Zentrum Geesthacht and the University of California, Berkeley, the research team led by Professor Gerold Schneider has discovered that nanoparticles can be arranged like atoms in a three-dimensional, periodic lattice, and adhere to each other with the help of ultra-thin layers of fatty acids. Since the nanoparticles are made of hard iron oxide, a type of rust, and the bonding layer is made of liquid oleic acid, the supercrystal is both hard and at the same time easy to deform, together with completely environmentally compatible. Perfect for surfaces subject to heavy wear.
New material concept
"Plastic deformation of materials such as copper, aluminum or steel has long been known in research. The fact that this mechanical behavior can also be transferred to high-strength supercrystals is completely new," explains Diletta Giuntini, a research associate at TU Hamburg and now an assistant professor at the Eindhoven University of Technology. "As part of our work, we have gained valuable knowledge about how to control the mechanical properties and deformability of supercrystals. In the next steps, we want to fine-tune their individual components and the interactions among them, and perfect these hybrid materials for a diverse set of applications," she continued.




























































