Plastics could see a second life as biodegradable surfactants
A new chemical process provides biodegradable chemicals
Advertisement
Scientists at the Institute for Cooperative Upcycling of plastics (iCOUP), an Energy Frontier Research Center led by Ames Laboratory, have discovered a chemical process that provides biodegradable, valuable chemicals, which are used as surfactants and detergents in a range of applications, from discarded plastics. The process has the potential to create more sustainable and economically favorable lifecycles for plastics.
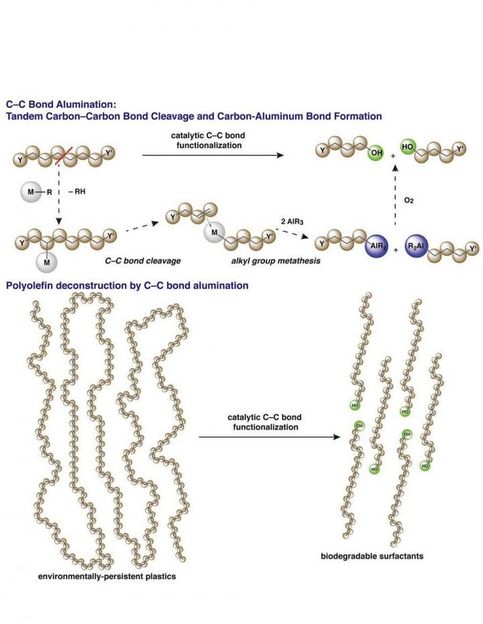
Long hydrocarbon chains of polymers are broken into shorter units with the introduction of aluminum end groups.
Ames Laboratory, U.S. Department of Energy
The researchers targeted their work on the deconstruction of polyolefins, which represents more than half of all discarded plastics, and includes nearly every kind of product imaginable-- toys, food packaging, pipe systems, water bottles, fabrics, shoes, cars, and furniture.
"Plastics, and especially polyolefins, are materials you could call too successful," said iCOUP Director Aaron Sadow. "They are fantastic-- strong, lightweight, thermally stable, chemically resistant-- for all the applications that we use them for, but the problem comes when we don't need them anymore."
It's all in the chemical construction of polyolefin plastics that makes them so tough and durable-- long strong chains of carbon-carbon bonds-- that also makes them hard to break down. Polyolefins also generally lack the chemical groups which could be targeted in deconstruction processes. Many existing processes to recycle plastic result in less-valuable, less usable components, making the economic feasibility of recycling far less appealing.
The new process uses what science already knows about key steps of polymerization-- the assembling of long polymer strands-- but in reverse, by breaking some of the carbon-carbon bonds in the chains. Once a few carbon-carbon bonds are broken, the shortened polymer chains transfer to an aluminum end group to form reactive species. The catalysts and reactions for this new process are related to those used in alkene polymerization, leveraging well-understood catalytic chemistry. Finally, the intermediates of this new transformation are easily converted into fatty alcohols or fatty acids, or used in other synthetic chemistry, to create chemicals or materials that are valuable in a whole host of ways: as detergents, emulsifiers, pharmaceuticals, and cosmetics. Because the process is catalytically controlled, desirable product chain lengths can be targeted for synthesis.
The best part about the process is that its end products are biodegradable, unlike polyethylene and polypropylene starting materials.
"Fatty acids and alcohols biodegrade in the environment relatively quickly. If these byproducts go on to find a new use elsewhere, that's wonderful, but it also has an end of life, which means it won't accumulate in the environment as plastics have," said Sadow.