Turning alcohols into alkylating agents
Taking a cue from nature
Advertisement
Researchers at Princeton have developed a dual catalyst system that directly installs alkyl groups onto heteroarenes. The new reaction uses simple and abundant alcohols and offers a milder and more widely applicable alternative to existing strategies.
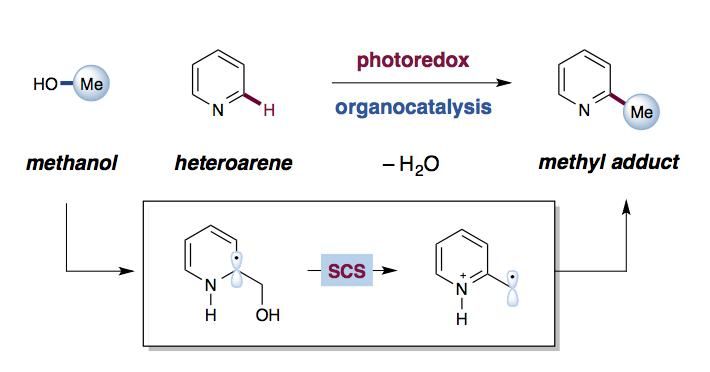
Alcohols as simple C-H alkylation agents via bio-inspired (spin center shift) process.
MacMillan group
The reported transformation is the first to successfully use alcohols as reagents in alkylation reaction. The unique reaction was actually discovered by chance, said David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry who led the work.
The research team was exploring alcohol additions when a test reaction revealed a surprising product that lacked the alcohol group. After further investigation, the team realized that the reaction underwent a well-known process in biochemistry, called spin center shift, which is involved in fundamental pathways such as DNA biosynthesis, but has rarely been seen in organic synthesis.
The researchers were able to mimic this natural phenomenon by using two types of catalysis in combination. The first catalyst, known as an iridium photocatalyst, initiates the reaction by taking a single electron from the second catalyst, a compound called a thiol, to form a key intermediate. That intermediate - a thiol radical - is uniquely capable of pulling a hydrogen atom off of the starting alcohol, cleaving a typically strong C-H bond. Breaking this bond reveals an alcohol species that reacts with the heteroarene and then follows the spin center shift pathway found in nature to form the final product.
Using their method, the researchers were able to synthesize a wide range of products, highlighting the incorporation of methyl groups. MacMillan and coworkers also demonstrated the efficient alkylation of two pharmaceutical compounds, showing its utility for late-stage alkylation.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
































































