A new breakthrough in the synthesis of chiral 3,6-Dihydro-2H-pyrans
Advertisement
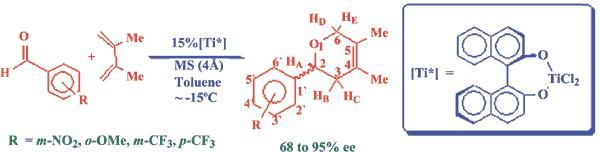
The pyran ring is present in so many useful compounds, such as pharmaceuticals (antibiotics, anti-infectives, cardiovascular agents, neurological modulators, anti-allergic, anti-asthmatic, anti-inflammatory agents, reproductive and genitourinary agents, growth promoters and antidiabetic agents), veterinary products, agrochemicals, toxins, polymers and additives, photosensitizers and photoinitiators, surfactants, food products, dyes and pigments, This fact keeps motivating synthetic organic chemists to develop newer facile synthetic methods to make these compounds accessible in high enantiomeric purity. Bansal and co-workers have recently succeeded in obtaining a series of phenyl substituted 3,6-Dihydro-2H-pyran derivatives in 68 to 95% enantiomeric excess.
The pyran ring is present in so many useful compounds.
Bansal et al, Bentham Science Publishers
This report has two important features. It illustrates that differently substituted 2-phenyl-3,6-dihydro-2H-pyrans can be obtained in high yield with high enantiomeric purity involving relatively simple experimental method. Secondly, the enantiomeric excess has been rationalized on the basis of computational calculations at the DFT level. It is hoped that the results would be useful for further research in this field.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.





























































