Shining a light on damage within polymers
Advertisement
When it comes to even the most advanced materials, the adage "if it does not bend, it breaks" is often true. But before that final snap, most materials experience microscopic damage that could be fixed -- but only if you know it's there. In a study researchers introduce a new technique that detects and illuminates damage in various types of materials.
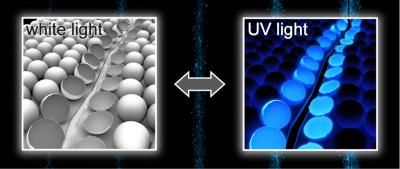
Embedded within polymers, microcapsules filled with aggregation-induced emission dyes break open when the surface is scratched; the resulting fluorescence allows researchers to detect the damage quickly and with high sensitivity.
American Chemical Society
Microscopic damage eventually leads to failure in polymers and composite materials, but it is difficult to detect without the aid of specialized equipment. Previously reported methods rely on chemical reactions or multicomponent systems to identify problems and are not generalizable over a wide range of applications and materials. In addition, many use color changes that are difficult to detect within the context of the objects in which they are embedded. To address these issues collectively, Jeffrey Moore, Nancy Sottos and colleagues envisioned a simple, single-component fluorescence system that would glow in response to microscopic damage.
The authors took advantage of a type of fluorescence called aggregation-induced emission (AIE). As opposed to most fluorescent dyes, the light emitted from AIE dyes becomes brighter as they solidify out of solution. To use AIE as a reporting strategy, the researchers created microcapsules containing solutions of AIE dyes and embedded them in a polymer. When they scratched the material's surface, the capsules broke and released the dye solution, causing the damaged region to glow blue under UV light as the liquid quickly evaporated. The researchers demonstrated that their method works for various materials and for different types of damage including a cut only 2 ?m wide, far smaller than could be seen with the naked eye. The authors propose that simple systems such as this one could reduce costs associated with routine inspection and pre-scheduled replacement of parts.





























































