Manganese may finally solve hydrogen fuel cells' catalyst problem
Widely available and inexpensive, the metal could lead to a renewable energy boom
Advertisement
manganese is known for making stainless steel and aluminum soda cans. Now, researchers say the metal could advance one of the most promising sources of renewable energy: hydrogen fuel cells.
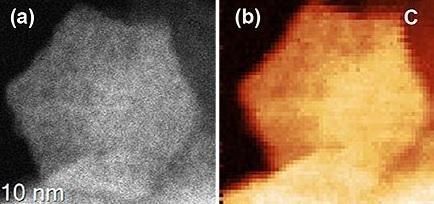
The image on the left shows manganese catalyst particle shape. The right images shows the uniform elemental distribution of carbon throughout the particle.
Gang Wu, University at Buffalo
In a study a University at Buffalo-led research team reports on catalysts made from the widely available and inexpensive metal.
The advancement could eventually help solve hydrogen fuel cells' most frustrating problem: namely, they're not affordable because most catalysts are made with platinum, which is both rare and expensive.
"We haven't been able to advance a large-scale hydrogen economy because of this issue involving catalysts. But manganese is one of the most common elements in Earth's crust and it's widely distributed across the planet. It could finally address this problem," says lead author Gang Wu, PhD, associate professor of chemical and biological engineering in UB's School of Engineering and Applied Sciences.
Additional authors come from Oak Ridge National Laboratory, Brookhaven National Laboratory, Argonne National Laboratory, Oregon State University, University of Pittsburgh, University of South Carolina, Giner Inc. and Harbin Institute of Technology.
For more than a decade, Wu has been searching for alternative catalysts for hydrogen fuel cells. He has reported advancements in iron- and cobalt-based catalysts; however, each wears down over time, limiting their usefulness, he says.
In previous work, Wu discovered that adding nitrogen to manganese causes internal changes to the metal that makes it a more stable element. In experiments reported in the study, he devised a relatively simple two-step method of adding carbon and a form of nitrogen called tetranitrogen to manganese.
The result was a catalyst that's comparable in its ability to split water -- the reaction needed to produce hydrogen -- as platinum and other metal-based alternatives. More importantly, the stability of the catalyst makes it potentially suitable for hydrogen fuel cells. This could lead to wide-scale adoption of the technology in buses, cars and other modes of transportation, as well as backup generators and other sources of power.
Wu plans to continue the research, focusing on improving the catalyst's carbon microstructure and the method in which nitrogen is added. The goal, he says, is to further enhance the catalyst's performance in practical hydrogen fuel cells.
Original publication
Jiazhan Li, Mengjie Chen, David A. Cullen, Sooyeon Hwang, Maoyu Wang, Boyang Li, Kexi Liu, Stavros Karakalos, Marcos Lucero, Hanguang Zhang, Chao Lei, Hui Xu, George E. Sterbinsky, Zhenxing Feng, Dong Su, Karren L. More, Guofeng Wang, Zhenbo Wang & Gang Wu; "Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells"; Nature Catalysis; 2018