Explosives at the microscopic scale produce shocking results
Advertisement
U.S. troops blew up enemy bridges with explosives in World War II to slow the advance of supplies or enemy forces. In modern times, patrollers use explosives at ski resorts to purposely create avalanches so the runs are safer when skiers arrive. Other than creating the desired effect (a destroyed bridge or avalanche), the users didn't exactly know the microscopic details and extreme states of matter found within a detonating high explosive. In fact, most scientists don't know what happens either.
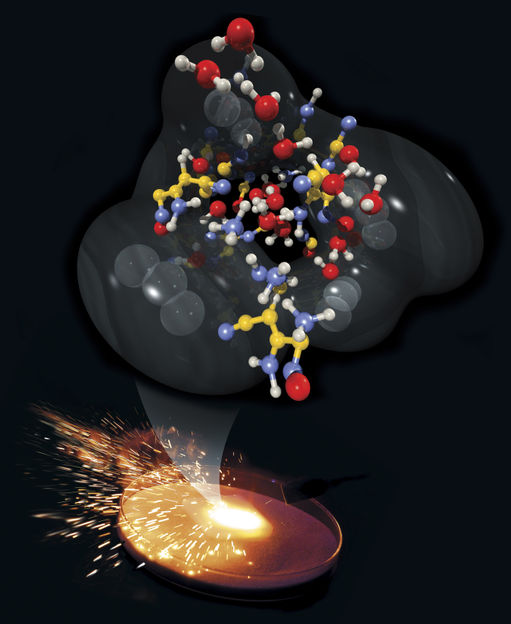
Sparks are emitted from rapid chemical reactions in an energetic material. This zoomed microscopic view shows a mixture of reaction intermediates observed during a computer simulation of detonating nitromethane.
Scott Dougherty
But researchers from Lawrence Livermore National Laboratory and the Massachusetts Institute of Technology have created the first quantum molecular dynamics simulation of a shocked explosive near detonation conditions, to reveal what happens at the microscopic scale. What they found is quite riveting: The explosive, nitromethane, undergoes a chemical decomposition and a transformation into a semi-metallic state for a limited distance behind the detonation front.
Nitromethane is a more energetic high explosive than TNT, although TNT has a higher velocity of detonation and shattering power against hard targets. Nitromethane is oxygen poor, but when mixed with ammonium nitrate can be extremely lethal, such as in the bombing of the Alfred P. Murrah Federal Building in Oklahoma City.
"Despite the extensive production and use of explosives for more than a century, their basic microscopic properties during detonation haven't been unraveled," said Evan Reed, the lead author of a paper appearing in Nature Physics. "We've gotten the first glimpse of the properties by performing the first quantum molecular dynamics simulation."
Nitromethane is burned as a fuel in drag racing autos, but also can be made to detonate, a special kind of burning in which the material undergoes a much faster and far more violent type of chemical transformation. With its single nitrogen dioxide (NO2) group, it is a simple representative version of explosives with more NO2 groups. Though it is an optically transparent, electrically insulating material, it undergoes a shocking transformation: It turns into an optically reflecting, nearly metallic state for a short time behind the detonation shock wave front. But further behind the wave front, the material returns to being optically transparent and electrically insulating.
"This is the first observation of this behavior in a molecular dynamics simulation of a shocked material," Reed said. "Ultimately, we may be able to create computer simulations of detonation properties of new, yet-to-be synthesized designer explosives."