To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Deuterium
Deuterium, also called heavy hydrogen, is a stable isotope of hydrogen with a natural abundance in the oceans of Earth of approximately one atom in 6500 of hydrogen (~154 PPM). Deuterium thus accounts for approximately 0.015% (on a weight basis, 0.030%) of all naturally occurring hydrogen in the oceans on Earth (see VSMOW; the abundance changes slightly from one kind of natural water to another). Deuterium abundance on Jupiter is about 6 atoms in 10,000 (0.06% atom basis)[1]; these ratios presumably reflect the early solar nebula ratios, and those after the Big Bang. There is little deuterium in the interior of the Sun, since thermonuclear reactions destroy it. However, it continues to persist in the outer solar atmosphere at roughly the same concentration as in Jupiter. The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common hydrogen nucleus contains no neutrons. The isotope name is formed from the Greek deuteros meaning "second", to denote the two particles comprising the nucleus.[2] Additional recommended knowledge
Differences between deuterium and common hydrogen (protium)Chemical symbolDeuterium is frequently represented by the chemical symbol D. Since it is an isotope of hydrogen with mass number 2, it is also represented by ²H. IUPAC allows both D and ²H, although ²H is preferred.[3] The reason deuterium has a distinct chemical symbol may be its large mass difference with protium (¹H); deuterium has a mass of 2.014 u, compared to the mean hydrogen atomic weight of 1.007947 u, and protium's mass of 1.007825 u. The isotope weight ratios within other chemical elements are largely insignificant in this regard, explaining the lack of unique isotope symbols elsewhere.[citation needed] Natural abundanceDeuterium occurs in trace amounts naturally as deuterium gas, written ²H2 or D2, but most natural occurrence in the universe is bonded with a typical ¹H atom, a gas called hydrogen deuteride (HD or ¹H²H).[4] The existence of deuterium on Earth, elsewhere in the solar system (as confirmed by planetary probes), and in the spectra of stars, is an important datum in cosmology. Stellar fusion destroys deuterium, and there are no known natural processes (for example, see the rare cluster decay), other than the Big Bang nucleosynthesis, which might have produced deuterium at anything close to the observed natural abundance of deuterium. This abundance seems to be a very similar fraction of hydrogen, wherever hydrogen is found. Thus, the existence of deuterium is one of the arguments in favor of the Big Bang theory over the steady state theory of the universe. The world's leading "producer" of deuterium (technically, merely enricher or concentrator of deuterium) was Canada, until 1997 when the last plant was shut down (see more in the heavy water article). Canada uses heavy water as a neutron moderator for the operation of the CANDU reactor design. India is now probably the world's largest concentrator of heavy water, also used in nuclear power reactors. Physical propertiesThe physical properties of deuterium compounds can be different from the hydrogen analogs; for example, D2O is more viscous than H2O.[citation needed] Deuterium behaves chemically similarly to ordinary hydrogen, but there are differences in bond energy and length for compounds of heavy hydrogen isotopes which are larger than the isotopic differences in any other element. Bonds involving deuterium and tritium are somewhat stronger than the corresponding bonds in light hydrogen, and these differences are enough to make significant changes in biological reactions (see heavy water). Deuterium can replace the normal hydrogen in water molecules to form heavy water (D2O), which is about 10.6% more dense than normal water (enough that ice made from it sinks in ordinary water). Heavy water is slightly toxic in eukaryotic animals, with 25% substitution of the body water causing cell division problems and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure). Prokaryotic organisms, however, can survive and grow in pure heavy water (though they grow more slowly). Consumption of heavy water would not pose a health threat to humans unless very large quantities (in excess of 10 liters) were consumed over many days. Small doses of heavy water (a few grams in humans, containing an amount of deuterium comparable to that normally present in the body) are routinely used as harmless metabolic tracers in humans and animals. Quantum propertiesThe deuteron has spin +1 and is thus a boson. The NMR frequency of deuterium is significantly different from common light hydrogen. Infrared spectroscopy also easily differentiates many deuterated compounds, due to the large difference in IR absorption frequency seen in the vibration of a chemical bond containing deuterium, versus light hydrogen. The two stable isotopes of hydrogen can also be distinguished by using mass spectrometry. Nuclear propertiesDeuterium is one of only four stable nuclides with an odd number of protons and odd number of neutrons. (2H, 6Li, 10B, 14N; also, the long-lived radioactive nuclides 40K, 50V, 138La, 180mTa occur naturally.) Most odd-odd nuclei are unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects. Deuterium, however, benefits from having its proton and neutron coupled to a spin-1 state, which gives a stronger nuclear attraction; the corresponding state does not exist in the two-neutron or two-proton system, due to the Pauli exclusion principle which would require one or the other particle to have some orbital angular momentum. But that would require orbital angular momentum and kinetic energy, so that they have a higher total energy (both due to their kinetic energy and because their distance would be larger and their binding energy lower). In both cases, this cases the di-proton and di-neutron nucleus to be unstable. Deuterium as an isospin singletDue to the similarity in mass and nuclear properties between the proton and neutron, they are sometimes considered as two symmetric types of the same object, a nucleon. While only the proton has an electric charge, this is often negligible due of the weakness of the electromagnetic interaction relative to the strong nuclear interaction. The symmetry relating the proton and neutron is known as isospin and denoted τ. Isospin is an SU(2) symmetry, like ordinary spin, so is completely analogous to it. The proton and neutron form an isospin doublet, with a "down" state A pair of nucleons can either be in an antisymmetric state of isospin called singlet, or in a symmetric state called triplet. In terms of the "down" state and "up" state, the singlet is This is a nucleus with one proton and one neutron, i.e. a deuterium nucleus. The triplet is
Approximated wavefunction of the deuteronThe total wavefunction of both the proton and neutron must be antisymmetric, because they are both fermions. Apart from their isospin, the two nucleons also have spin and spatial distributions of their wavefunction. The latter is symmetric if the deuteron is symmetric under parity (i.e. have an "even" or "positive" parity) , and antisymmetric if the deuteron is antisymmetric under parity (i.e. have an "odd" or "negative" parity). The parity is fully determined by the total orbital angular momentum of the two nucleons: if it is even then the parity is even (positive), and if it is odd then the parity is odd (negative). The deuteron, being an isospin singlet, is antisymmetric under nucleons exchange due to isospin, and therefore must be symmetric under the double exchange of their spin and location. Therefore it can be in either of the following two different states:
In the first case the deuteron has is a Spin triplet, so that its total spin s is 1. It also has an even parity and therefore even orbital angular momentum l ; The lower its orbital angular momentum, the lower its energy. Therefore the lowest possible energy state has s =1, l =0. In the second case the deuteron has is a spin singlet, so that its total spin s is 0. It also has an odd parity and therefore odd orbital angular momentum l . Therefore the lowest possible energy state has s =0, l =1. Since s =1 gives a stronger nuclear attraction, the deuterium ground state is in the s =1, l =0 state. The same considerations lead to the possible states of an isospin triplet having s =0, l =even or s =1, l =odd. Thus the state of lowest energy has s =1, l =1, higher than that of the isospin singlet. The analysis just given is in fact only approximate, both because isospin is not an exact symmetry, and more importantly because the strong nuclear interaction between the two nucleons is related to angular momentum in a way that mixes different s and l states. That is, s and l are not constant in time (they do not commute with the Hamiltonian), and over time a state such as s =1, l =0 may become a state of s =1, l =2. Parity is still constant in time so these do not mix with odd l states (such as s =0, l =1). Therefore the quantum state of the deuterium is a superposition (a linear combination) of the s =1, l =0 state and the s =1, l =2 state, even though the first component is much bigger. Since the total angular momentum j is also a good quantum number (it is a constant in time), both components must have the same j, and therefore j =1. This is the total spin of the deuterium nucleus. To summarize, the deuterium nucleus is antisymmetric in terms of isospin, and has spin 1 and even (+1) parity. The relative angular momentum of its nucleons l is not well defined, and the deuterium is a superposition of mostly l =0 with some l =2. Magnetic and electric multipolesIn order to find theoretically the deuterium magnetic dipole moment μ, one uses the formula for a nuclear magnetic moment with g(l) and g(s) are g-factors of the nucleons. Since the proton and neutron have different values for g(l) and g(s), one must separate their contributions. Each gets half of the deuterium orbital angular momentum where subscripts p and n stand for the proton and neutron, and g(l)n = 0. By using the same identities as here and using the value g(l)p = 1 in nuclear magneton units, we arrive at the following result, in nuclear magneton units For the s =1, l =0 state j =1 and we get, in nuclear magneton units For the s =1, l =2 state with j =1 we get, in nuclear magneton units The measured value of the deuterium magnetic dipole moment, in nuclear magneton units, is 0.857. This suggests that the state of the deuterium is indeed only approximately s =1, l =0 state, and is actually a linear combination of (mostly) this state with s =1, l =2 state. The electric dipole is zero as usual. The measured electric quadropole of the deuterium is 0.2859 e fm², where e is the proton electric charge and fm is fermi. While the order of magnitude is reasonable, since the deuterium radius is of order of 1 fermi (see below) and its electric charge is e, the above model does not suffice for its computation. More specifically, the electric quadropole does not get a contribution from the l =0 state (which is the dominant one) and does get a contribution from a term mixing the l =0 and the l =2 states, because the electric quadrupole operator does not commute with angular momentum. The latter contribution is dominant in the absence of a pure l =0 contribution, but cannot be calculated without knowing the exact spatial form of the nucleons wavefunction inside the deuterium. Higher magnetic and electric multipole moments cannot be calculated by the above model, for similar reasons. Deuterium radius
The square root of the average squared radius of the deuterium, measured experimentally, is
ApplicationsDeuterium is useful in nuclear fusion reactions, especially in combination with tritium, because of the large reaction rate (or nuclear cross section) and high energy yield of the D-T reaction. There is an even higher-yield D-He3 fusion reaction, though the breakeven point of D-He3 is higher than that of most other fusion reactions; together with the scarcity of He3, this makes it implausible as a practical power source until at least D-T and D-D fusion reactions have been performed on a commercial scale. Unlike protium, deuterium undergoes fusion purely via the strong interaction, making its use for commercial power plausible. In chemistry and biochemistry, deuterium is used as a non-radioactive isotopic tracer in molecules to study chemical reactions and metabolic pathways, because chemically it behaves similarly to ordinary hydrogen, but it can be distinguished from ordinary hydrogen by its mass, using mass spectrometry or infrared spectrometry. Neutron scattering techniques particularly profit from availability of deuterated samples: The H and D cross sections are very distinct and different in sign, which allows contrast variation in such experiments. Further, a nuisance problem of ordinary hydrogen is its large incoherent neutron cross section, which is nil for D and delivers much clearer signals in deuterated samples. Hydrogen occurs in all materials of organic chemistry and life science, but cannot be seen by X-ray diffraction methods. Hydrogen can be seen by neutron diffraction and scattering, which makes neutron scattering, together with a modern deuteration facility, indispensable for many studies of macromolecules in biology and many other areas. Deuterium is useful in hydrogen nuclear magnetic resonance spectroscopy (proton NMR). NMR ordinarily requires compounds of interest to be analyzed as dissolved in solution. Because of deuterium's nuclear spin properties which differ from the light hydrogen usually present in organic molecules, NMR spectra of hydrogen/protium are highly differentiable from that of deuterium, and in practice deuterium is not "seen" by an NMR instrument tuned to light-hydrogen. Deuterated solvents (including heavy water, but also compounds like deuterated chloroform CDCl3) are therefore routinely used in NMR spectroscopy, in order to allow only the light-hydrogen spectra of the compound of interest to be measured, without solvent-signal interference. Deuterium can also be used for femtosecond infrared spectroscopy, since the mass difference drastically affects the frequency of molecular vibrations; deuterium-carbon bond vibrations are found in locations free of other signals. Measurements of small variations in the natural abundances of deuterium, along with those of the stable heavy oxygen isotopes 17O and 18O, are of importance in hydrology, to trace the geographic origin of Earth's waters. The heavy isotopes of hydrogen and oxygen in rainwater (so-called meteoric water) are enriched as a function of the environmental temperature of the region in which the precipitation falls (and thus enrichment is related to mean latitude). The relative enrichment of the heavy isotopes in rainwater (as referenced to mean ocean water), when plotted against temperature falls predictably along a line called the global meteoric water line (GMWL). This plot allows samples of precipitation-originated water to be identified along with general information about the climate in which it originated. Evaporative and other processes in bodies of water, and also ground water processes, also differentially alter the ratios of heavy hydrogen and oxygen isotopes in fresh and salt waters, in characteristic and often regionally-distinctive ways.[5] The proton and neutron making up deuterium can be dissociated through neutral current interactions with neutrinos. The cross section for this interaction is comparatively large, and deuterium was successfully used as a neutrino target in the Sudbury Neutrino Observatory experiment. HistoryLighter element isotopes suspectedThe existence of nonradioactive isotopes of lighter elements had been suspected in studies of neon as early as 1913, and proven by mass spectroscopy of light elements in 1920. The prevailing theory at the time, however, was that the isotopes were due to the existence of differing numbers of "nuclear electrons" in different atoms of an element. It was expected that hydrogen, with a measured average atomic mass very close to 1 u, and a nucleus thought to be composed of a single proton (a known particle), could not contain nuclear electrons, and thus could have no heavy isotopes. Deuterium predicted and finally detectedDeuterium was predicted in 1926 by Walter Russell, using his "spiral" periodic table. It was first detected spectroscopically in late 1931 by Harold Urey, a chemist at Columbia University. Urey's collaborator, Ferdinand Brickwedde, distilled five liters of cryogenically-produced liquid hydrogen to 1 mL of liquid, using the low-temperature physics laboratory that had recently been established at the National Bureau of Standards in Washington, DC (now the National Institute of Standards and Technology). This concentrated the fraction of the mass-2 isotope of hydrogen to a degree that made its spectroscopic identification unambiguous; Urey called the isotope "deuterium" from the Greek and Latin words for "two." The amount inferred for normal abundance of this heavy isotope was so small (only about 1 atom in 6400 hydrogen atoms in ocean water) that it had not noticeably affected previous measurements of (average) hydrogen atomic mass. Urey was also able to concentrate water to show partial enrichment of deuterium. Gilbert Newton Lewis prepared the first samples of pure heavy water in 1933. The discovery of deuterium, coming before the discovery of the neutron in 1932, was an experimental shock to theory, and after the neutron was reported, deuterium won Urey the Nobel Prize in chemistry in 1934. "Heavy water" experiments in World War IIShortly before the war, Hans von Halban and Lew Kowarski moved their research on neutron moderation from France to England, smuggling the entire global supply of heavy water (made in Norway) across in twenty-six steel drums.[6][7] During World War II, Nazi Germany was known to be conducting experiments using heavy water as moderator for a nuclear reactor design. (Heavy water is water in which the hydrogen is deuterium.) Such experiments were a source of concern because they might allow them to produce plutonium for an atomic bomb. Ultimately, it led to (what seemed to be important at that time) the Allied operation called the "Norwegian heavy water sabotage," the purpose of which was to destroy the Vemork deuterium production/enrichment facility in Norway. After World War II ended, the Allies discovered that Germany was not putting as much serious effort into the program as has had been previously thought. The Germans had completed only a small, partly-built experimental reactor (which had been hidden away). By the end of the war, the Germans did not even have a fifth the amount of heavy water needed to run the reactor, partially due to the Norwegian heavy water sabotage operation. However, even had the Germans succeeded in getting a reactor operational (as the U.S. did with a graphite reactor in late 1942), they would still have been at least several years away from development of an atomic bomb with maximal effort. The engineering process, even with maximal effort and funding, required about two and a half years (from first critical reactor to bomb) in both the U.S. and U.S.S.R, for example (see the article heavy water for a more complete history of its production and use). Data
Data at approximately 18 K for D2 (triple point):
Anti-deuteriumAn antideuteron is the antiparticle of the nucleus of deuterium, consisting of an antiproton and an antineutron. The antideuteron was first produced in 1965 at the Proton Synchrotron at CERN[8] and the Alternating Gradient Synchrotron at Brookhaven National Laboratory[9]. A complete atom, with a positron orbiting the nucleus, would be called antideuterium, but as of 2005 antideuterium has not yet been created. The symbol for antideuterium is the same as for deuterium, except with a bar over it. See alsoReferencesNotes
General references
Categories: Isotopes of hydrogen | Environmental isotopes | Neutron moderators | Nuclear fusion fuels |
|||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Deuterium". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||





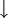 being a
being a 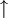 being a
being a 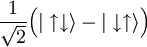
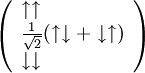 And thus consists of three types of nuclei, which are supposed to be symmetric - a deuterium nucleus (actually a highly excited state of it), a nucleus with two
And thus consists of three types of nuclei, which are supposed to be symmetric - a deuterium nucleus (actually a highly excited state of it), a nucleus with two 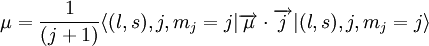
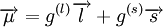
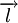 and spin
and spin 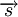 . One arrives at
. One arrives at
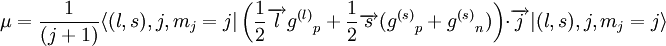
![\mu = {1\over 4 (j+1)}\left[({g^{(s)}}_p + {g^{(s)}}_n)\big(j(j+1) - l(l+1) + s(s+1)\big) + \big(j(j+1) + l(l+1) - s(s+1)\big)\right]](images/math/4/9/f/49f653501f931fbf9f1c67276a9ec4e4.png)
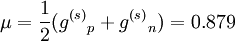
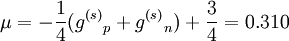
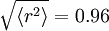 fermi (= 0.96 fm).
fermi (= 0.96 fm).


