Not so fast: Some batteries can be pushed too far
Scientists find overabundance of intentional defects can cause battery cathodes to fail
Advertisement
Intentional defects in batteries have given Rice University scientists a window into the hazards of pushing lithium-ion cells too far.
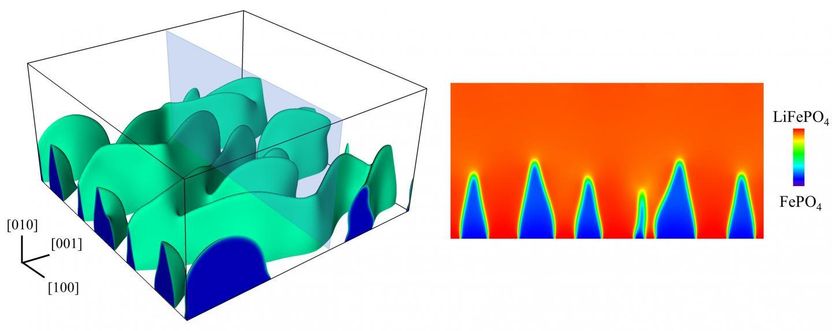
At left, a 3D model by Rice University materials scientists shows a phase boundary as a delithiating lithium iron phosphate cathode undergoes rapid discharge. At right, a cross-section shows the "fingerlike" boundary between iron phosphate (blue) and lithium (red). Rice engineers found that too many intentional defects intended to make batteries better can in fact degrade their performance and endurance.
Mesoscale Materials Science Group/Rice University
New simulations by Rice materials scientist Ming Tang and graduate student Kaiqi Yang, detailed in the Journal of Materials Chemistry A, shows too much stress in widely used lithium iron phosphate cathodes can open cracks and quickly degrade batteries.
The work extends recent Rice research that demonstrated how putting defects in particles that make up the cathode could improve battery performance by up to two orders of magnitude by helping lithium move more efficiently.
But the lab's subsequent modeling study revealed a caveat. Under the pressure of rapid charging and discharging, defect-laden cathodes risk fracture.
"The conventional picture is that lithium moves uniformly into the cathode, with a lithium-rich region that expands smoothly into the cathode's center," said Tang, an assistant professor of materials science and nanoengineering at Rice's Brown School of Engineering.
But X-ray images taken at another lab showed something else. "They saw a fingerlike boundary between the lithium-rich and lithium-poor regions, almost like when you inject water into oil," he said. "Our question was, what causes this?"
The root of the problem appears to be that stress destabilizes the initially flat boundary and causes it to become wavy, Tang said. The change in the boundary shape further increases the stress level and triggers crack formation. The study by Tang's group shows that such instability can be increased by a common type of defect in battery compounds called antisites, where iron atoms occupy spots in the crystal where lithium atoms should be.
"Antisites can be a good thing, as we showed in the last paper, because they accelerate the lithium intercalation kinetics," Tang said, "But here we show a countereffect: Too many antisites in the particles encourage the moving interface to become unstable and therefore generate more stress."
Tang believes there's a sweet spot for the number of antisites in a cathode: enough to enhance performance but too few to promote instability. "You want to have a suitable level of defects, and it will require some trial and error to figure out how to reach the right amount through annealing the particles," he said. "We think our new predictions might be useful to experimentalists."
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.