Scientists succeeds in producing dodecacene for the first time
World record in research on π-electron structures
Advertisement
A team of international scientists led by of Francesca Moresco (Center for Advancing Electronics Dresden – cfaed at TU Dresden) and Diego Peña Gil (Center for Research in Biological Chemistry and Molecular Materials – CiQUS at University of Santiago de Compostela) has achieved a breakthrough in the field of π-electron structures research. For the first time, they synthesised a chain of twelve benzene rings, called dodecacene, which is the longest acene ever obtained to date. This was made possible by using on-surface synthesis and observed by scanning tunneling microscopy. The investigation of the properties of higher acenes also revealed an unexpected increase in the energy gap of dodecacene.
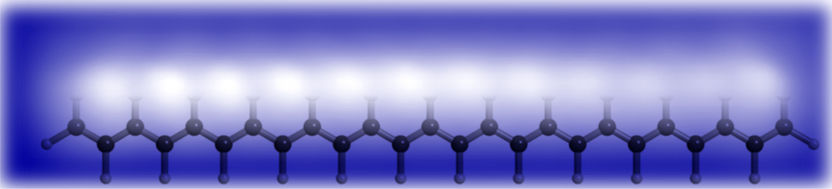
STM image and schematic representation of dodecacene overlap in this figure. Twelve bright lobes, representing the 12 benzene rings, are well distinguishable.
© cfaed der TU Dresden
An international team of researchers from TU Dresden, University of Santiago de Compostela, and CEMES-CNRS Institute in Toulouse has succeeded in synthesising a chain of twelve benzene rings, the so-called dodecane by on-surface deoxygenation of a stable precursor molecule. Using scanning tunnelling microscopy and spectroscopy, the researchers also examined the electronic properties of dodecacenes. Surprisingly, their experiments showed that the energy gap remains constant for decacenes and undecacenes, but increases again for dodecacene. This phenomenon is particularly interesting for future research in molecular electronics and spintronics.
What are acenes and why are higher acenes so relevant but complicated?
Acenes are organic compounds, more precisely polycyclic aromatic hydrocarbons consisting of a varying number of linear fused benzene rings. The acene series represents a model system to investigate the intriguing electronic properties of extended π-electron structures in the one-dimensional limit, which are important for applications in electronics and spintronics and for the fundamental understanding of electronic transport.
Especially higher acene series are currently of great interest in research because of their special electronic properties. Recent research efforts suggest a higher radical character as well as the stabilisation of the optical excitation energy for an increasing number of secured benzene rings, which is very attractive for nanoelectronic devices. However, they are difficult to synthesise, because they are chemically very reactive, therefore unstable, and not soluble.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.































































