Digitizing chemistry with a smart stir bar
A magnetic stirrer bar with an integrated process monitoring system
Advertisement
Miniaturized computer systems and wireless technology are offering scientists new ways to keep tabs on reactions without the need for larger, cumbersome equipment. In a proof-of-concept study in ACS Sensors, researchers describe an inexpensive new device that functions like a conventional magnetic stir bar, but that can automatically measure and transmit information on a solution's color, viscosity and a variety of other attributes to a smart phone or computer.
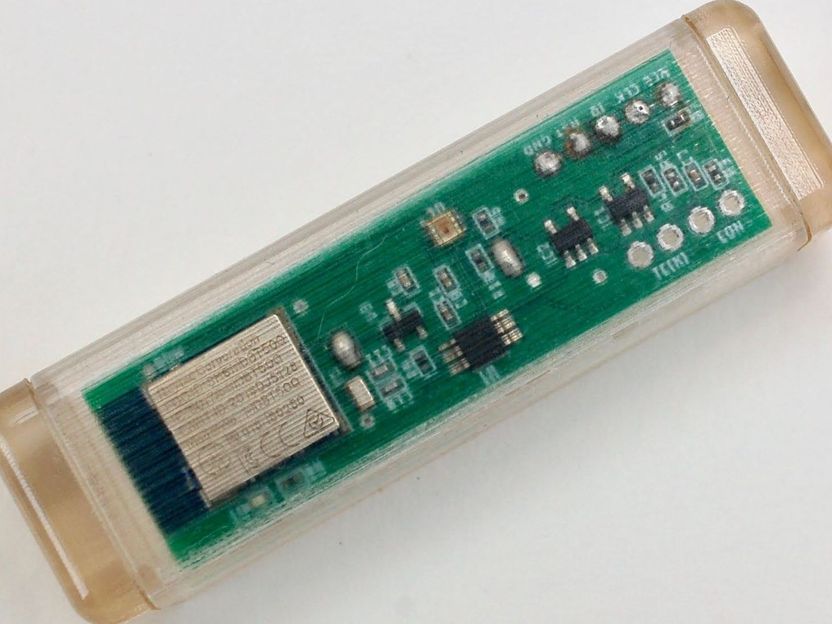
The Smart Stirrer can transmit data on the color, viscosity and other attributes of the solution it is stirring.
Dmitry Isakov
Automatic, remote data collection can make chemical processes more reliable, as well as less labor intensive and safer. This technology has begun to make inroads into chemistry labs, but options for scientists looking to remotely mix their reactions and monitor several parameters while those reactions occur remain quite scarce, limited and expensive. Nikolay Cherkasov, Dmitry Isakov and colleagues wanted to develop a device capable of simultaneously detecting numerous parameters using freely available open source software and easy-to-get, inexpensive components.
The team made two versions of the Smart Stirrer based on different integrated circuits, one a microcontroller and the other an all-in-one system on a chip. To customize the Smart Stirrer for different applications, the team used a modular design, in which sensors can be added as needed. In experiments, they confirmed they could detect color, electrical conductivity and, with careful calibration, viscosity. Additional low-cost commercially available sensors, as well as custom ones, could be used to adapt the device for many new applications, the researchers say. They note one limitation: the narrow range of temperatures within which the Smart Stirrer can function, a restriction that they say is inherent to most digital electronics. Ultimately, they predict that this approach could provide a platform for digitizing chemistry in research labs and industrial manufacturing.
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.