Heat-Insulating Titanate
Discovering new leads for functional materials guided by artificial intelligence
Advertisement
Searching for lead materials with specific properties, researchers have developed a workflow that incorporates artificial intelligence to guide discovery of a new ceramic structure with particularly low thermal conductivity. As they explain in the journal Angewandte Chemie, the material has an unusual quasicrystalline structure, potentially paving the way for novel heat-insulating and thermoelectric materials.
© Wiley-VCH
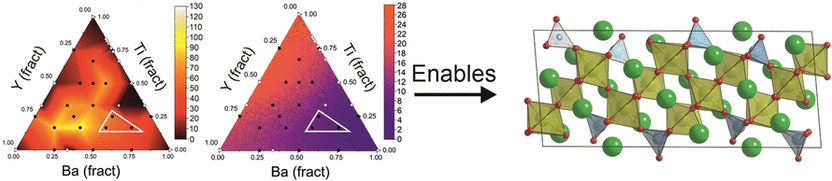
Ceramics with low thermal conductivity are constantly being sought for heat protection coatings or thermoelectric applications. In other words, for generating current from heat. Matthew J. Rosseinsky of the University of Liverpool, UK, and his colleagues, took the chemical group of the titanates as their starting point in this search. Based on energy calculations, they concentrated their search on titanates containing fractions of yttrium and barium oxides.
To narrow down candidates with compositions which would give a material with potentially even lower thermal conductivity, the researchers turned to artificial intelligence, training machine learning models with ceramics of known composition and known thermal conductivity. The models confirmed their original knowledge-based decision to limit themselves to barium–yttrium titanates.
The AI results also showed that the composition can have further impact on the thermal conductivity. “This guided us to prefer one of the two composition regions identified by the energy calculations for experimental work,” Rosseinsky says. Thus, the researchers synthesized a new oxide, as yet unknown, composed of ten parts barium, six parts yttrium, four parts titanium, and 27 parts oxygen atoms.
The new material turned out to be metastable, and its structure proved to be particularly surprising. In “normal” crystals, atoms are arranged periodically. However, in the new material the team observed a “quasicrystalline” structure. Quasicrystals have an ordered arrangement of atoms, but not full three-dimensional periodicity. Only when quasicrystals are considered in terms of “long-range order” can the continuous periodicity typical of crystals be recognized. The team highlighted the significance of these findings: “Oxide quasicrystals have been observed at interfaces, however, the material presented here is the first that has been proposed as a quasicrystal in the bulk.”
The new titanate was found to have a lower thermal conductivity than almost all other known transition metal oxides of this type, with only one molybdenum oxide with a complex crystal structure giving better results. The authors also explained the thermal conductivity of their material in theoretical terms, comparing the behavior of the quasicrystal to that of glass. Glasses have an unordered material structure, and are known to be good thermal insulators.
The team emphasized the role of deploying an integrated set of tools, based on knowledge and understanding of chemistry, and incorporating machine learning models. “Our study shows how AI can help in decision making to accelerate discovery,” Rosseinsky says.