Light Instead of Electricity: A New Kind of “Green Hydrogen”
Simple and promising
Advertisement
Today, green hydrogen is usually created by splitting up water using electrical current derived from renewable energy sources. Scientists at TU Wien have now developed a new photocatalyst design that could make this process more direct and controllable.
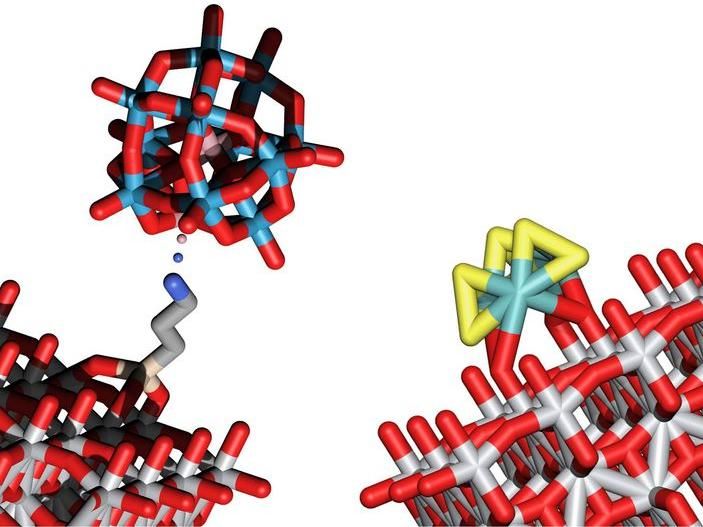
Structural models of two clusters that enable water splitting into O2 and H2 by means of light energy
Technische Universität Wien
Hydrogen could be an important part of our future energy supply: It can be stored, transported and burned as needed. However, most of the hydrogen available today is a by-product of natural gas production - this has to change for climate protection reasons. The best strategy so far to produce environmentally friendly "green hydrogen" is to split water into hydrogen and oxygen using electricity that comes from renewable energy sources, for example photovoltaic cells.
However, it would be much easier if sunlight could be used directly to split water. This is exactly what new catalysts are now making possible, in a process called "photocatalytic water splitting". The concept not yet used industrially. At TU Wien, important steps have now been taken in this direction: on an atomic scale, scientists realized a new combination of molecular and solid-state catalysts that can do the job relying only on relatively inexpensive materials.
Interaction of different atoms
"Actually, to be able to split water with light you have to solve two tasks at the same time" says Alexey Cherevan from the Institute for Materials Chemistry at TU Wien. "We have to think about oxygen and about hydrogen. The oxygen atoms of the water must be transformed into O2 molecules, and the remaining hydrogen ions – which are just protons – must be turned into H2 molecules.”
Solutions have now been found for both tasks: Tiny inorganic clusters consisting of only a small number of atoms are anchored on a surface of light-absorbing support structures such as titanium oxide. The combination of clusters and carefully chosen semiconductor supports leads to the desired behavior.
The clusters responsible for oxidising oxygen are made up of cobalt, tungsten and oxygen, while clusters of sulphur and molybdenum are especially suitable for creating hydrogen molecules. The researchers at TU Wien were the first to deposit these clusters on a surface made of titanium oxide, where they can act as catalysts for water splitting.
"Titanium oxide is sensitive to light, that was already well known," says Alexey Cherevan. "The energy of the absorbed light leads to the creation of free-moving electrons and free-moving positive charges in the titanium oxide. These charges then allow the clusters of atoms sitting on this surface to facilitate the splitting of water into oxygen and hydrogen."
Precise control, atom by atom
"Other research groups working on splitting water with light rely on nanoparticles that can take on very different shapes and surface properties," explains Alexey Cherevan. "The sizes are hard to control, the atoms are not quite arranged in the same way. Therefore, in this case, it is not possible to explain exactly how the catalysis process takes place in detail." At TU Wien, on the other hand, the exact structure of the clusters is determined with atomic precision, which allows to gain full understanding of the catalytic cycle.
"This is the only way to get feedback on what the efficiency of the process really depends on," says Alexey Cherevan. "We don't want to just rely on a trial and error approach and try different nanoparticles until we find the best one - we want to find out at the atomic level what the optimal catalyst really is."
Now that it has been proven that the selected materials are indeed suitable for splitting water, the next step is to further tune their exact structure to achieve even higher efficiencies.
Simple and promising
"The decisive advantage of our method over splitting water by electrolysis is its simplicity," Alexey Cherevan emphasises. Electric hydrogen production first needs a sustainable energy source - such as photovoltaic cells, possibly an electric energy storage device and an electrolysis cell. All in all, this results in a relatively complex system consisting of a multitude of raw materials. For photocatalytic water splitting, on the other hand, all that is needed is a suitably coated surface that is covered by water and irradiated by the sun.
In the long term, this knowledge could also be used to produce more complicated molecules using the concept of artificial photosynthesis. It might even be possible to use the energy of solar radiation to produce hydrocarbons with carbon dioxide from the atmosphere and water, which can then be used for other applications.
Original publication
S. Batool et al., Surface-Anchoring and Active Sites of [Mo3S13]2- Clusters as Co-Catalysts for Photocatalytic Hydrogen Evolution. ACS Catalysis, 2022, 12, 6641-6650.
S.P. Nandan et al., Immobilization of a [CoIIICoII(H2O)W11O39]7- Polyoxoanion for Photocatalytic Oxygen Evolution Reaction, ACS Materials Au, 2022, just accepted.