Photochemical Turbo Power for Solar Cells
How organic molecules make yellow light from red
Advertisement
Modern silicon solar cells achieve a maximum efficiency of around 25 percent. Researchers around the world are running a race to increase this efficiency even further. However, a natural limit exists at around 30 percent because the laws of physics prevent solar cells from absorbing light of energies lower than a certain material-specific limit. The energy of this light is lost; it is not turned into electricity. Scientists at the University of Sydney and of Helmholtz Zentrum Berlin (HZB) have now demonstrated in lab experiments a way to avoid these losses.
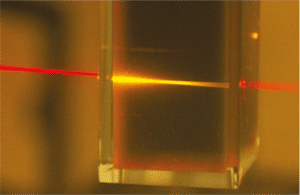
Red light from a laser pointer is converted into higher-energy yellow light as it passes through the liquid photochemical upconverter.
University of Sydney, Australia
They have developed a kind of “turbo for solar cells”, called photochemical upconversion: Two energy-poor photons that would normally be ineffective in the solar cell are merged into one energy-rich photon, which can then contribute towards the electricity yield. Further research in this direction may make it possible to exceed the 30 percent mark.
The photochemical solar-cell turbo uses organic molecules to merge energy-poor red photons together into energy-richer yellow photons. The secret is in the choice of molecules, of which two different types are placed behind the solar cell in solution. The task of the first molecule type is to absorb the energy-poor light particles and to store their energy. The crux here is that these molecules enter a persistent state in which the spins, or magnetic moments, of the light-excited electrons in each molecule line up in parallel. This prevents re-emission of the absorbed particles.
This persistent state of the first molecule type lasts long enough for the energy to be transferred into a persistent state of a second type of organic molecule. This energy transfer takes place when the two types of molecule encounter each other in the solution. If two excited molecules of the second type then encounter each other, then one of them returns to its base state. The other thereupon assumes an even higher energy state, which is extremely short-lived. This latter molecule then sends off a single photon of high enough energy to be absorbed by the solar cell.
“We are thus the first to demonstrate an efficiency gain in a solar cell by photochemical upconversion,” says project head Dr. Klaus Lips of the HZB Institute for Silicon Photovoltaics. “The achieved increase in solar cell efficiency is still low – about 0.1 percent absolute – and the sunlight even had to be concentrated fifty times, but the path to further improvement is clearly discernible.” Yet it is rocky and hard, as Lips emphasizes: “For the concept study now published, we had not used a 25 percent high-capacity solar cell yet, as will be needed for later practical application.” They now have to redevelop the organic molecules of the photochemical upconverter so that they do not have to be dissolved in a liquid. They will also have to perform their action under normal, unconcentrated sunlight, and an infrared converter will be required for crystalline silicon.
“The concepts for this were developed in close cooperation between Sydney and HZB,” says Klaus Lips. The essential advantage of this ‘3rd generation photovoltaics’ over other approaches is there is no need for costly redevelopment of solar cells; rather, merely adding the upconverter would in principle suffice to boost the efficiency. Klaus Lips concludes: “Just as you would build a turbo into a car to make it go faster – and wouldn’t necessarily go and design an entirely new car.”





























































