Open-Sesame Time for Nanocapsules
Nanocavity skeleton altered by substitution of metal ions
Advertisement
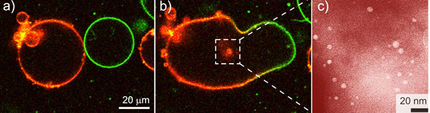
Hollow spherical capsules composed of pentagonal molybdenum-oxide units stitched together by metal-ion linkers are a remarkable example of porous nanoscale objects, and have attracted much recent attention because of their potential applications in areas as diverse as catalysis and biosensing. These clusters are similar in design to soccer balls with up to twelve highly charged pentagonal units linked together by multiple metal cations, thus defining a distinct internal cavity. Unlike other hollow objects, these nanocapsules have very well-defined external and internal surfaces, and indeed the surfaces can be modified to interact with a desired species. Now a team of researchers from Bielefeld University, Max Planck Institute of colloids and Interfaces, University of Ulm, and the University of Stuttgart, along with their collaborators at Lehigh University, have come up with a method to access the internal nanocavities of these capsules.
Achim Müller, Tianbo Liu, and their colleagues have found that when the iron cations holding the capsule together are partially substituted by other metal ions (such as lanthanide ions), the capsule momentarily opens because of the decomposition of some of the constituent pentagonal units. This partial opening of the capsule allows the entry of smaller molybdate species and lanthanide ions into the nanocavity.
The soccer-ball framework of the capsules is stabilized by the iron ions; however, these ions can be easily exchanged for other metal cations. The researchers believe that upon the loss of the linking ions, the highly charged molybdenum-oxide pentagons can no longer be stabilized in the capsule skeleton and thus decompose into smaller molybdate fragments. This creates an opening in the capsule and allows the flow of material from the external solution to the nanocavity. The formed molybdate fragments enter the internal space to compensate the charges on the capsule skeleton and are distributed randomly within the cavity. Finally, the new cations replace the iron linkers and the external/spherical skeleton is reconstituted.
"It will be interesting to see if this method can be used to encapsulate other interesting species within the capsules", said Müller, adding that these types of capsules are also useful to model the transport of biological cations across membranes. Liu pointed out that the incorporation of fluorescent lanthanide ions in the capsule skeleton makes these structures promising for biosensing applications. The researchers also believe that ordered assemblies of filled nanocapsules may exhibit hitherto unseen collective properties.
Original publication: Achim Müller et al.; "Nanometer-Sized Molybdenum-Iron Oxide Capsule-Surface Modifications: External and Internal"; Small 2007.
Most read news
Topics
Organizations
Other news from the department science
These products might interest you

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.