Chromium replaces rare and expensive noble metals
Advertisement
Expensive noble metals often play a vital role in illuminating screens or converting solar energy into fuels. Now, chemists at the University of Basel have succeeded in replacing these rare elements with a significantly cheaper metal. In terms of their properties, the new materials are very similar to those used in the past.
State-of-the-art chromium compounds act as luminescent materials and catalysts
Universität Basel, Jo Richers
We’re familiar with chromium from everyday applications such as chromium steel in the kitchen or chrome-plated motorcycles. Soon, however, the element may also be found in the screens of ubiquitous mobile phones or used to convert solar energy. Researchers led by Professor Oliver Wenger from the Department of Chemistry at the University of Basel have developed chromium compounds that can replace the noble metals osmium and ruthenium — two elements that are almost as rare as gold or platinum — in luminescent materials and catalysts. Writing in Nature Chemistry, the team reports that the luminescent properties of the new chromium materials are nearly as good as some of the osmium compounds used so far. Relative to osmium, however, chromium is about 20,000 times more abundant in the earth’s crust — and much cheaper.
The new materials are also proving to be efficient catalysts for photochemical reactions, including processes that are triggered by exposure to light, such as photosynthesis. Plants use this process to convert energy from sunlight into energy-rich glucose and other substances that serve as fuel for biological processes.
If the new chromium compounds are irradiated with a red lamp, the energy from the light can be stored in molecules which can then serve as a power source. “Here, there’s also the potential to use our new materials in artificial photosynthesis to produce solar fuels,” explains Wenger.
Tailor-made packaging for chromium
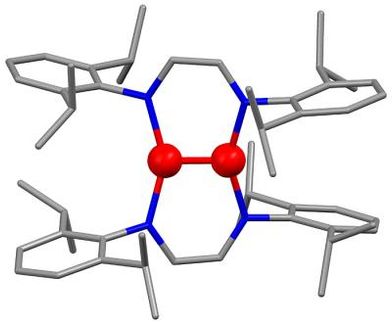
To make the chromium atoms glow and enable them to convert energy, the researchers built them into an organic molecular framework consisting of carbon, nitrogen, and hydrogen. The team designed this organic framework to be particularly stiff, so that the chromium atoms are well packaged. This tailor-made environment helps to minimize energy losses due to undesired molecular vibrations and to optimize the luminescent and catalytic properties. The disadvantage of the new materials is that chromium requires a more complex framework than noble metals — and further research will therefore be needed in the future.
Encased in its rigid organic framework, chromium proves to be much more reactive than noble metals when exposed to light. This paves the way for photochemical reactions that are otherwise difficult to initiate. A potential application could be in the production of active pharmaceutical ingredients.
Competition with other alternatives
For a long time, the search for sustainable and cost-effective materials without noble metals focused primarily on iron and copper. Other research groups have already achieved promising results with both of these elements, and chromium has also been incorporated into luminescent materials in the past.
In many cases, however, the luminescent and catalytic properties of these materials lagged far behind those of materials containing rare and expensive noble metals — therefore failing to represent a real alternative. The new materials made of chromium are different because they contain a form of chromium that is particularly similar to noble metals, thereby achieving luminescent and catalytic efficiencies that come very close to materials containing such metals.
“At the moment, it seems unclear which metal will ultimately win the race when it comes to future applications in luminescent materials and artificial photosynthesis,” says Wenger. “What is certain, however, is that the postdocs Dr. Narayan Sinha and Dr. Christina Wegeberg have made important progress together.”
Next, Wenger and his research group aim to develop their materials on a larger scale to allow broader testing of potential applications. By making additional improvements, they hope to achieve light emission in different spectral colors from blue to green to red. They also want to further optimize the catalytic properties in order to bring us a major step closer to converting sunlight into chemical energy for storage — as in photosynthesis.