Watching catalysts at work -- at the atomic scale
Innovative combination of methods at HZB leads to fundamental insights in catalyst research
Advertisement
Scientists of Helmholtz-Zentrum Berlin (HZB) and collaborators have now combined the spectroscopic method "RIXS" with so-called ab initio theory in order to describe these processes in detail for a model organometallic catalyst of great interest to catalysis research – the iron carbonyl complex. The team publishes its results today in the prestigious scientific journal "Angewandte Chemie International Edition".
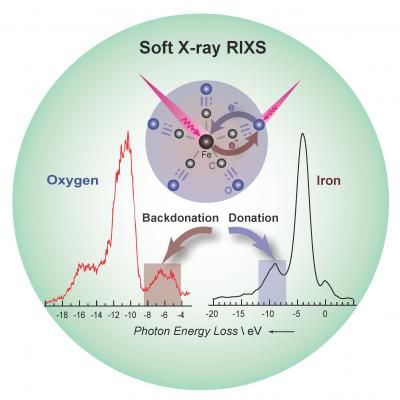
Fundamental processes: Charge donation/backdonation in the [Fe(CO)5] model catalyst in solution was studied by resonant inelastic X-ray scattering. This method can be used to selectively probe the electronic structure at each atom in the iron-carbonyl bond.
HZB/E. Suljoti
Iron carbonyl complexes are used in a large number of chemical reactions and industrial processes, such as light-induced water reduction or catalytic carbon monoxide removal from exhaust gases. Their catalytic activity is a result of rapid formation and subsequent breaking of chemical bonds between the metal centre and the carbonyl ligands. "It is essential for us to be able to determine the strength of orbital mixing at the chemical bond by directly probing the metal centres and the ligands," says Prof. Dr. Emad Flear Aziz, head of the HZB junior research group 'Structure and Dynamics of Functional Materials'. Until recently, has not been possible to apply these studies in homogeneous catalysis which take place in solution. The development of the new "LiXEdrom" experimental station, here at HZB, which is equipped with the micro-jet technique has enabled RIXS (resonant inelastic X-ray scattering) experiments on functional materials under in-situ conditions.
In collaboration with scientists from various universities, Aziz's team has now successfully studied both the metal and the ligands under real conditions in which this particular catalysis takes place (in situ), using RIXS spectroscopy at HZB's electron storage ring BESSY II. They discovered a very strong orbital mixing between the metal and its ligands, which led to a weakening and elongation of the chemical bond during RIXS excitation. The experimental results were supported by theoretical ab initio methods by the University of Rostock. "With this new method combination, we have gained fundamental insights into the electronic structure of iron carbonyl complexes under catalysis-relevant conditions," Aziz reports. "Our approach can help provide a better understanding of reaction dynamics and metal-ligand-solvent interactions on very short time scales. This leads to better control of catalytic properties – and holds great potential for the production of novel catalytically active materials."





























































