Fixing by Filtration
New testing strips for detecting heavy metals
Advertisement
Many heavy metals are toxic to the environment or to humans. Legal limits for these pollutants in drinking water and run-off are thus correspondingly low. Rapid on-the-spot analysis and routine water quality tests demand a rapid, cost-effective method that doesn't require complex instruments. Test strips that indicate the presence and concentration of a heavy metal when simply dipped into the water are ideal. Commercially available strips are currently not sufficiently reliable or sensitive. Japanese researchers have now developed a new generation of test strips that meet these high demands. Their secret is pigment nanocrystals that are fixed to a membrane filter by means of simple filtration.
One of the main problems of current testing strips for heavy metals is that the reagents can be washed out, which significantly reduces their effectiveness. A truly satisfactory process for the fixation of pigments was previously unknown. A research team headed by Toshishige M. Suzuki has now discovered a very simple method for fixing the heavy-metal-specific color reagents to the test strips so that they can neither be washed away by the test solution nor rubbed off. They were thus able to produce testing strips that react specifically to divalent zinc, mercury, and iron.
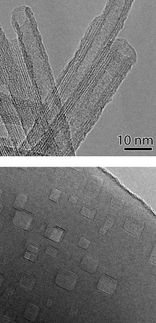
The color reagent must first be formed into nanoscopic particles. The pigment is thus dissolved in an organic solvent and then sprayed into vigorously stirred water. Because the pigment is not water-soluble, it crystallizes out, and under these conditions it crystallizes in the form of nanocrystals that are finely dispersed in the solution. When filtered through a cellulose membrane, 99.5% of the nanoparticles stick to the membrane surface in a fine, dense, even layer. This precise control of the amount of pigment applied is an important requirement for testing strips that give reproducible results. The continuous layer of the color reagent makes the strips highly sensitive, allowing the detection of zinc ions at concentrations as low as 65 ppb (parts per billion). Lower detection limits can be achieved by the filtration of larger amounts of the test solution through the reagent-coated membrane.
This new method for the production of testing strips can be used for many water-insoluble color reagents, allowing for the development of a large family of metal-specific testing strips.
Original publication: T. M. Suzuki; "Test Strips for Heavy-Metal Ions Fabricated from Nanosized Dye Compounds"; Angewandte Chemie International Edition 2005.
Topics
Organizations
Other news from the department science
These products might interest you

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.