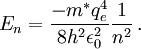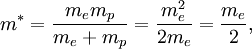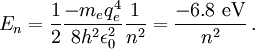To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
PositroniumPositronium (Ps) is a system consisting of an electron and its anti-particle, a positron, bound together into an "exotic atom". The orbit of the two particles and the set of energy levels is similar to that of the hydrogen atom (electron and proton). However, because of the lower reduced mass, the frequencies associated with the spectral lines are less than half of those of the corresponding hydrogen lines. Product highlight
StatesThe ground state of positronium, like that of hydrogen, has two possible configurations depending on the relative orientations of the spins of the electron and the positron. The singlet state with antiparallel spins (S = 0, Ms = 0) is known as para-positronium (p-Ps) and denoted 1S0. It has a mean lifetime of 125 picoseconds and decays preferably into two gamma quanta with energy of 511 keV each (in the center of mass frame). Detection of these photons allows for the reconstruction of the vertex of the decay and is used in the positron emission tomography. Para-positronium can decay into even number of photons (2, 4, 6,...), but the probability quickly decreases: the branching ratio for decay into 4 photons is 1.439(2)×10−6 [1]. The triplet state with parallel spins (S = 1, Ms = −1, 0, 1) is known as ortho-positronium (o-Ps) and denoted ³S1. The triplet state in vacuum has a mean lifetime of 142.05±0.02 nanoseconds[2] and the leading mode of decay is three gamma quanta. Other modes of decay are negligible; for instance, the five photons mode has branching ratio of ~1.0×10−6 [3]. Positronium in the 2S state is metastable having a lifetime of 1.1 μs against annihilation.[citation needed] If the positronium is created in such an excited state then it will quickly cascade down to the ground state where annihilation will occur more quickly. Measurements of these lifetimes, as well as of the positronium energy levels, have been used in precision tests of quantum electrodynamics. [4][1] Annihilation can proceed via a number of channels each producing one or more gamma rays. The gamma rays are produced with a total energy of 1022 keV (since each of the annihilating particles have mass of 511 keV/c²), the most probable annihilation channels produce two or three photons, depending on the relative spin configuration of the electron and positron. A single photon decay is only possible if another body (e.g. an electron) is in the vicinity of the annihilating positronium to which some of the energy from the annihilation event may be transferred. Up to five annihilation gamma rays have been observed in laboratory experiments, confirming the predictions of quantum electrodynamics to very high order. The annihilation into a neutrino-antineutrino pair is also possible, but the probability is predicted to be negligible. The branching ratio for o-Ps decay for this channel is 6.2×10−18 (electron neutrino-antineutrino pair) and 9.5×10−21 (for each non-electron flavour) [3] in predictions based on the Standard Model, but it can be gained by non-standard neutrino properties, like mass or relatively high magnetic moment. The experimental upper limits on branching ratio for this decay are: <1.7×10−6 (p-Ps) and <2.8×10−6 (o-Ps) [5]. Energy levels
The similarity between positronium and hydrogen extends even to the equation that gives a rough estimate of the energy levels. The energy levels are different between the two because of a different value for the mass, m*, used in the energy equation
The reduced mass in this case is
Thus, for positronium, its reduced mass only differs from the rest mass of the electron by a factor of 2. This causes the energy levels to also roughly be half of what they are for the hydrogen atom. So finally, the energy levels of positronium are given by The lowest energy level of positronium (n = 1) is −6.8 electron volts (eV). The next highest energy level (n = 2) is −1.7 eV. The negative sign implies a bound state. Observation of di-positronium moleculesThe first observation of di-positronium molecules — molecules consisting of two positronium atoms — was reported on 12 September 2007 by David Cassidy and Allen Mills from University of California at Riverside.[6][7] Prediction and discoveryCroatian scientist Stjepan Mohorovičić predicted of the existence of positronium in 1934, in a paper published in Astronomische Nachrichten, in which he called the substance "electrum".[8] Other sources credit Carl Anderson as having predicted its existence in 1932 while at Caltech.[9] It was experimentally discovered by Martin Deutsch at MIT in 1951, and became known as positronium.[9] References
See alsoCategories: Subatomic particles | Molecular physics | Exotic atoms |
|||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Positronium". A list of authors is available in Wikipedia. | |||||||||||||||