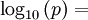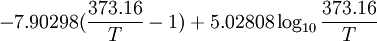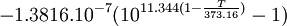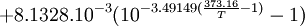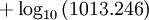To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Water vapor
Water vapor or water vapour (see spelling differences), also aqueous vapor, is the gas phase of water. Water vapor is one state of the water cycle within the hydrosphere.[2] Water vapor can be produced from the evaporation of liquid water or from the sublimation of ice. Under normal atmospheric conditions,[3] water vapor is continuously evaporating and condensing. Product highlight
General properties of water vaporEvaporation/sublimationWhenever a water molecule leaves a surface, it is said to have evaporated. Each individual water molecule which transitions from a more associated (liquid) to a less associated (vapor/gas) state does so through the absorption or release of kinetic energy. The aggregate measurement of this kinetic energy transfer is defined as thermal energy and occurs only when there is differential in the temperature of the water molecules. Liquid water that becomes water vapor takes a parcel of heat with it, in a process called evaporative cooling.[4] The amount of water vapor in the air determines how fast each molecule will return back to the surface. When a net evaporation occurs, the body of water will undergo a net cooling directly related to the loss of water.[5] Evaporative cooling is restricted by atmospheric conditions. The amount of water vapor in the air is the humidity. The vapor content of air is measured with devices known as hygrometers. The measurements are expressed as specific humidity or percent relative humidity. The temperatures of the atmosphere and the water surface determine the equilibrium vapor pressure; 100% relative humidity occurs when the partial pressure of water vapor is equal to the equilibrium vapor pressure. This condition is often referred to as complete saturation. Another form of evaporation is sublimation, by which water molecules become gaseous directly from ice without first becoming liquid water. When ice has a higher temperature than the surrounding atmosphere, sublimation occurs. Sublimation accounts for the slow mid-winter disappearance of ice and snow at temperatures too low to cause melting. CondensationWater vapor will only condense onto another surface when that surface is cooler than the temperature of the water vapor, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface.[6] The water molecule brings a parcel of heat with it. In turn, the temperature of the atmosphere drops slightly.[7] [8] In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by cloud condensation nuclei). The dew point of an air parcel is the temperature to which it must cool before water vapor in the air begins to condense. Also, a net condensation of water vapor occurs on surfaces when the temperature of the surface is at or below the dew point temperature of the atmosphere. Deposition, the direct formation of ice from water vapor, is a type of condensation. Frost and snow are examples of deposition. Water vapor densityWater vapor is lighter or less dense than dry air. At equivalent temperatures it is buoyant with respect to dry air. Water vapor and dry air density calculations at 0°CThe molecular mass or weight of water is 18.02g/mol, as calculated from the sum of the atomic masses of its constituent atoms. The average molecular mass of air (Approx. 79% nitrogen, N2; 21% Oxygen, 02) is 28.57g/mol at standard temperature and pressure (STP). Using Avogadro's Law and the ideal gas law, water vapor and air will have a molar volume of 22.414 litre/mol at STP. A molar mass of air and water vapour occupy the same volume of 22.414 litres. The density (mass/volume) of water vapor is 0.804g/litre, which is significantly less than that of dry air at 1.27g/litre at STP. Note that STP conditions include a temperature of 0°C, at which the ability of water to become vapor is very restricted. Its concentration in air is very low at 0°C. The red line on the chart to the right is the maximum concentration of water vapor expected for a given temperature. The water vapor concentration increases significantly as the temperature rises, approaching 100% (steam, pure water vapor) at 100°C. However the difference in densities between air and water vapour would still exist. Air and water vapor density interactions at equal temperaturesAt the same temperature, a column of dry air will be denser or heavier than a column of air containing any water vapor. Thus, any volume of dry air will sink if placed in a larger volume of moist air. Also, a volume of moist air will rise or be buoyant if placed in a larger region of dry air. As the temperature rises the proportion water vapor in the air increases, its buoyancy will become larger. This increase in buoyancy can have a signicant atmospheric impact, giving rise to powerful, moisture rich, upward air currents when the air temperature and sea temperature reachs 25°C or above. This phenomenon provides a significant motivating force for cyclonic and anticyconic weather systems (tornados and hurricanes). Water vapour and respiration or breathingWater vapor's contribution to the pressure increases as its concentration increases. Its partial pressure contribution to air pressure increases, lowering the partial pressure contribution of the other atmospheric gases (Dalton's Law). The total air pressure must remain constant. The presence of water vapor in the air naturally dilutes or displaces the other air components as its concentration increases. This can have an effect on respiration, in very warm air (35°C). The proportion of water vapor is significant enough to give rise to the stuffiness that can be experienced in humid jungle conditions or in badly air conditioned buildings. General discussionThe amount of water vapor in an atmosphere is constrained by the restrictions of partial pressures and temperature. Dew point temperature and relative humidity act as guidelines for the process of water vapor in the water cycle. Energy input, such as sunlight, can trigger more evaporation on an ocean surface or more sublimation on a chunk of ice on top of a mountain. The balance between condensation and evaporation gives the quantity called vapor partial pressure[9]. The maximum partial pressure (saturation pressure) of water vapor in air varies with temperature of the air and water vapor mixture. A variety of empirical formulas exist for this quantity; the most used reference formula is the Goff-Gratch equation for the SVP over liquid water:
The formula is valid from about −50 to 102 °C; however there are a very limited number of measurements of the vapor pressure of water over supercooled liquid water.[10] Under adverse conditions, such as when the boiling temperature of water is reached, a net evaporation will always occur during standard atmospheric conditions regardless of the percent of relative humidity. This immediate process will dispel massive amounts of water vapor into a cooler atmosphere. Exhaled air is almost fully at equilibrium with water vapor at the body temperature. In the cold air the exhaled vapor quickly condenses, thus showing up as a fog or mist of water droplets and as condensation or frost on surfaces. Controlling water vapor in air is a key concern in the heating, ventilating, and air-conditioning (HVAC) industry. Thermal comfort depends on the moist air conditions. Non-human comfort situations are called refrigeration, and also are affected by water vapor. For example many food stores, like supermarkets, utilize open chiller cabinets, or food cases, which can significantly lower the water vapor pressure (lowering humidity). This practice delivers several benefits as well as problems. Water vapor in Earth's atmosphereGaseous water represents a small but environmentally significant constituent of the atmosphere. Approximately 99.99% of it is contained in the troposphere. The condensation of water vapor to the liquid or ice phase is responsible for clouds, rain, snow, and other precipitation, all of which count among the most significant elements of what we experience as weather. Less obviously, the latent heat of vaporization, which is released to the atmosphere whenever condensation occurs, is one of the most important terms in the atmospheric energy budget on both local and global scales. For example, latent heat release in atmospheric convection is directly responsible for powering destructive storms such as tropical cyclones and severe thunderstorms. Water vapor is also a potent greenhouse gas. Because the water vapor content of the atmosphere is expected to greatly increase in response to warmer temperatures, there is the potential for a water vapor feedback that could amplify the expected climate warming effect due to increased carbon dioxide alone. However, it is less clear how cloudiness would respond to a warming climate; depending on the nature of the response, clouds could either further amplify or partly mitigate the water vapor feedback. Fog and clouds form through condensation around cloud condensation nuclei. In the absence of nuclei, condensation will only occur at much lower temperatures. Under persistent condensation or deposition, cloud droplets or snowflakes form, which precipitate when they reach a critical mass. The average residence time of water molecules in the troposphere is about 10 days. Water depleted by precipitation is replenished by evaporation from the seas, lakes, rivers and the transpiration of plants, and other biological and geological processes. Measurements of vapor concentration are expressed as specific humidity or percent relative humidity. The annual mean global concentration of water vapor would yield about 25 mm of liquid water over the entire surface of the Earth if it were to instantly condense. However, the mean annual precipitation for the planet is about 1 meter, which indicates a rapid turnover of water in the air. The abundance of gases emitted by volcanoes varies considerably from volcano to volcano. However, water vapor is consistently the most common volcanic gas, normally comprising more than 60% of total emissions during a subaerial volcanic eruption.[11] Radar and satellite imagingBecause water molecules absorb microwaves and other radio wave frequencies, water in the atmosphere attenuates radar signals.[12] In addition, atmospheric water will reflect and refract signals to an extent that depends on whether it is vapor, liquid or solid.[13] Generally, radar signals lose strength progressively the farther they travel through the troposphere. Different frequencies attenuate at different rates, such that some components of air are opaque to some frequencies and transparent to others. Radio waves used for broadcasting and other communication experience the same effect. Water vapor reflects radar[14] to a less extent than do water's other two phases. In the form of drops and ice crystals, water acts as a prism, which it does not do as an individual molecule; however, the existence of water vapor in the atmosphere causes the atmosphere to act as a giant prism.[15] A comparison of GOES-12 satellite images shows the distribution of atmospheric water vapor relative to the oceans, clouds and continents of the Earth. Vapor surrounds the planet but is unevenly distributed. Lightning generationWater vapor plays a key role in lightning production in the atmosphere. From cloud physics, usually, clouds are the real generators of static charge as found in Earth's atmosphere. But the ability, or capacity, of clouds to hold massive amounts of electrical energy is directly related to the amount of water vapor present in the local system. The amount of water vapor directly controls the permittivity of the air. During times of low humidity, static discharge is quick and easy. During times of higher humidity, fewer static discharges occur. However, permittivity and capacitance[16] work hand in hand to produce the megawatt outputs of lightning. After a cloud, for instance, has started its way to becoming a lightning generator, atmospheric water vapor acts as a substance (or insulator[17] [18] ) that decreases the ability of the cloud to discharge its electrical energy. Over a certain amount of time, if the cloud continues to generate and store[19] more static electricity[20], the barrier that was created by the atmospheric water vapor will ultimately break down[21] from the stored electrical potential energy. This energy will be released to a locally, opposite[22] charged region in the form of lightning. The strength of each discharge is directly related to the atmospheric permittivity, capacitance, and the source's charge generating ability.[23] See also, Van de Graaff generator. Extraterrestrial water vaporThe brilliance of comet tails comes largely from water vapor. On approach to the sun, the ice many comets carry sublimates to vapor, which reflects light from the sun. Knowing a comet's distance from the sun, astronomers may deduce a comet's water content from its brilliance.[24] Bright tails in cold and distant comets suggests carbon monoxide sublimation. Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.[25] Most of the water on Mars appears to exist as ice at the northern pole. During Mars' summer, this ice sublimates, perhaps enabling massive seasonal storms to convey significant amounts of water toward the equator.[26] A star called CW Leonis was found to have a ring of vast quantities of water vapor circling the aging, massive star. A NASA satellite designed to study chemicals in interstellar gas clouds, made the discovery with an onboard spectrometer. Most likely, "the water vapor was vaporized from the surfaces of orbiting comets."[27] Spectroscopic analysis of HD 209458 b, an extrasolar planet in the constellation Pegasus, provides the first evidence of atmospheric water vapor beyond the Solar System. Scientific Discrepancies, Confounding factors and limits of knowledgeWater Vapour being practically ubiquitous, has been studied and written about from many perspectives. As a working knowledge has grown and developed within apparently unrelated fields several discrepancies in understanding may be encountered. These discrepancies often arise from an inability to rigidly determine either a Volumetric or Gravimetric basis of study ; and/or use of constants inappropriate for the conditions being observed. Many scienific studies view water vapour as a Confounding variable (preventing Ceteris paribus, also 'lurking varible') due to its complex nature, this becomes especially true when the study observes significant variation in water vapor quantities, over time and/or location. It is for the reasons above that this remains a particularly tricky and sometime controversial factor in many fields of science, whether storage of foods or ancient artefacts, Thermodynamics or Climate Change. See also
Footnotes/References
Categories: Atmospheric thermodynamics | Forms of water | Psychrometrics |
|||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Water_vapor". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||