Chemists develop new multi-component reaction
More environmentally friendly approach produces complex molecular structures in one step: Ketyl radicals "tamed" by photocatalysis
Advertisement
A more sustainable use of chemical resources is part of the United Nations' Agenda 2030. Synthetic chemists are therefore working to design and carry out efficient syntheses. Within the synthetic organic chemist's arsenal, processes that link several molecules (coupling partners) in a single step - so-called multi-component reactions (MCRs) - play a central role. These are considered sustainable and environmentally friendly technologies for the rapid production of complex structures and drugs in a single reaction step. A team of researchers led by chemistry professor Frank Glorius (University of Münster, Germany) and Dr Huan-Ming Huang (University of Münster and ShanghaiTech University, China) has now succeeded for the first time in using so-called ketyl radicals for a novel MCR. This study was published in the recently founded journal Nature Synthesis.
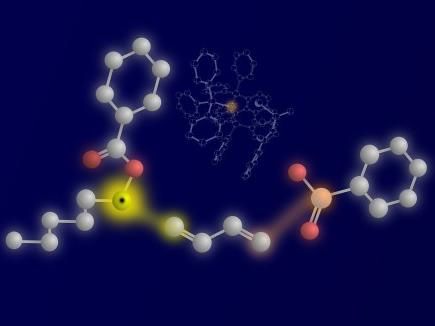
The three reactants and their linkage: ketyl-type radical on the left, 1,3-diene on the center, and the nucleophile sulfinate on the right. The reactive atoms and the bond that are being formed are highlighted in yellow (ketyl carbon and first C―C bond) and orange (Sulfur nucleophile atom and second C―S bond). The catalytically active palladium complex, with the palladium center highlighted in orange, is visible in the background.
© WWU – Peter Bellotti
"Ketyl radicals are very important species in synthetic chemistry. They are often used in the synthesis of complex natural-occurring products. However, catalytic chemical transformations that use ketyl radicals remain challenging. Their formation often requires 'harsh' reaction conditions with a high temperature. The radicals can also be non-selective in their reaction pathways, which means they are difficult to control," explains Huan-Ming Huang.
The research team used ketyl radicals and palladium catalysis excited by visible light to create an MCR between several coupling partners. When selecting the coupling partners (aldehydes, 1,3-dienes and various nucleophiles), the researchers took various aspects into account: Which substances are necessary for the reaction to take place, which are readily available and which products are useful?
"We succeeded in taming ketyl-type radicals by combining visible light with small amounts of a commercially available palladium catalyst," emphasizes co-author Peter Bellotti. "This operationally simple, redox-neutral and thus environmentally friendly approach could become a general platform for the construction of so-called complex homoallylic alcohol motifs, a frequently used structural motif in synthetic chemistry. The one-step synthesis of key intermediates that can be further converted to valuable products is proof of the versatility of this approach."
In addition to the synthetic capabilities of this method, the team investigated the mechanistic intricacies using a combined experimental mechanistic analysis and density functional theory (DFT) calculations. "We anticipate that the combination of visible light with transition metals such as palladium could inspire further unforeseen synthetic transformations beyond the established catalysed reactions," Frank Glorius sums up.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.































































