Charged “molecular beasts” the basis for new compounds
Researchers use “aggressive” fragments of molecular ions for chemical synthesis
Advertisement
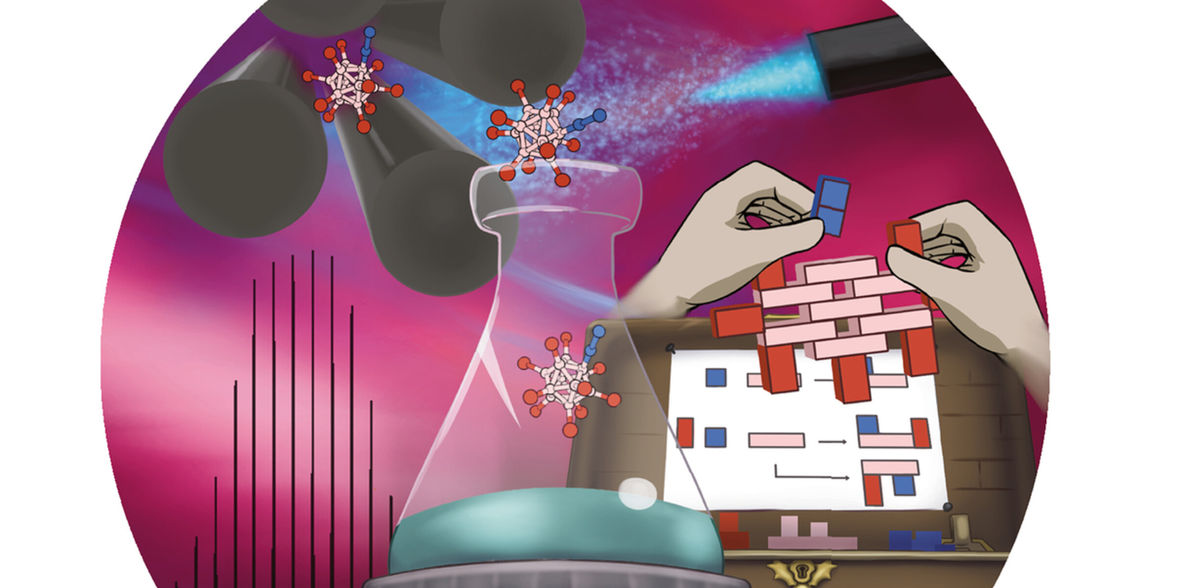
mass spectrometers are high-tech machines that play an important role in our society. They are highly sensitive analytical instruments that are indispensable in areas such as medical diagnostics, food quality control and the detection of hazardous chemical substances. The research group led by Dr Jonas Warneke at the Wilhelm-Ostwald-Institute for Physical and Theoretical Chemistry at Leipzig University is working to modify mass spectrometers so that they can be used for a completely different purpose: the chemical synthesis of new molecules. These preparative mass spectrometers can be used to produce chemical compounds in a new way. The researchers recently synthesised a new compound from a charged molecular fragment and nitrogen from the air, which has a wide range of potential applications in building new molecular structures. They have published their new findings in the journal “Angewandte Chemie”. The journal cover visualizes the concept of “harvesting” molecules that were composed from fragments in a seed box-like approach in the gas phase of a mass spectrometer directly into a chemical flask that would typically be employed for conventional synthesis.

Cover of the journal Angewandte Chemie.
Leipzig University
Developing new ways to break and reform chemical bonds is one of the main tasks of basic chemical research. “When a bond in a charged molecule is broken, the result is often a chemically ‘aggressive’ fragment, which we call a reactive fragment. These fragments are difficult to control using established methods of chemical synthesis. You can think of them as untamed beasts that attack anything in their path. In a mass spectrometer, there are many ways to break certain bonds and generate fragments,” says Dr Warneke, describing the processes in mass spectrometers. According to him, the “beasts” are kept under special conditions because there is a vacuum inside the mass spectrometer. This means that there is nothing for them to attack, thus preventing uncontrolled chemical reactions. “If we then offer a certain molecule, for example nitrogen, which is normally unreactive and doesn’t bind, the beast is satisfied with it because it has no other choice,” he says. In this way, molecules that are very difficult to bind, such as nitrogen, can be easily incorporated into a new substance,” Warneke continues.
In the past, the research team has used this approach to bring reactive fragments into very unusual reactions, for example, with noble gases, which are the most difficult of all chemical elements to bind. “The basic strategy of controlling chemical beasts in mass spectrometers is not new,” says Warneke. It has been used for decades to analyse the properties of reactive fragments. However, the new compounds found in this way could not be further used. Mass spectrometers show what is happening inside them, but the new substances are only produced in tiny quantities and cannot usually be extracted. They are often simply destroyed when the signal used for analyses is generated.
This is why researchers usually come away from experiments with mass spectrometers with “great knowledge” but “empty hands”. “They have the beast under control. Exactly what they were hoping for happens, they observe the new molecule with potentially fascinating properties, and then it’s gone,” says Warneke, describing chemical experiments in conventional mass spectrometers. The new publication could fundamentally change this view of chemical reactions in mass spectrometers. The research team produced a new substance from an aggressive fragment and unreactive nitrogen and collected it with preparative mass spectrometers in sufficient quantities so that it could be seen with the naked eye, handled and further experimented with.
The amount of substance produced by this method will remain limited to thin film technology applications for some time to come. However, preparative mass spectrometry could soon open up completely new possibilities for these applications, for example, in the production of microchips, solar cells or biologically active coatings. The junior research group has now reached an important milestone in its project, which has been funded by the Volkswagen Foundation’s Freigeist Fellowship since 2020.