Chemists design new molecule, with oxygen as the star of the show
New design rules for capturing a stable, chiral oxygen atom
Advertisement
Colorado State University chemists have achieved a new feat in the realm of chemical design and synthesis: They’ve helped create the first example of a synthetic molecule, with an asymmetric oxygen atom as its centerpiece, that remains stable and nonreactive – despite this type of molecule’s tendency in nature to be touchy and short-lived.
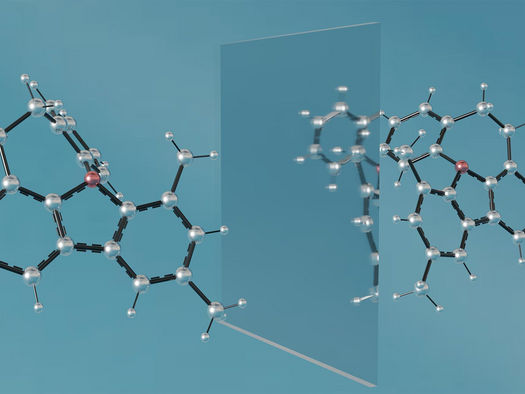
Illustration of the molecule CSU researchers helped design, together with its mirror image.
Mihai Popescu/Colorado State University
What makes this feat unique is that the new molecule is chiral, which means it has a non-superimposable mirror image. Chiral molecules, which can be thought of as compounds with “left-” and “right-” handed versions, have long fascinated chemists because while they resemble each other, they can have drastically different properties. For example, limonene is a chiral molecule with two different mirror-image forms; one has the characteristic smell of oranges, and the other smells like lemons. In clinical environments, mirror-image forms of drug molecules can have divergent, even deleterious effects.
Robert Paton, professor in the Department of Chemistry, and Mihai Popescu, a postdoctoral researcher in Paton’s lab, worked on this project with collaborators at the University of Oxford, and their work was recently published in the journal Nature. Paton and Popescu led theoretical and computational studies that established new design rules for capturing a stable, chiral oxygen atom. This allowed their colleagues at Oxford to pursue the synthesis and analysis of these molecules, which are triaryl oxonium ions that can be isolated at room temperature.
Chiral molecules with carbon as their centerpiece have been widely explored, but in this study, the researchers used oxygen instead of carbon. No one had ever achieved this before, because oxygen atoms in naturally occurring chiral molecules, which are called oxoniums, tend to flip between their mirror-image forms; this makes them very reactive with their surroundings. This reactivity makes chiral oxonium molecules very difficult to synthesize and isolate in the laboratory.
With this work, the researchers have gifted molecular designers a new tool in their arsenal, chiral oxonium, much like a new type of building material with potentially unique properties. From drug discovery to materials engineering, the new oxonium could open up a whole new chapter in chemistry by design.
“The discovery of a fundamentally new example of molecular chirality demonstrates our ability as chemists to design and synthesize new matter based on a computational blueprint,” Paton said. “Given the fundamental importance of chirality in catalysis, medicine and materials, it will be exciting to explore the properties of chiral oxygen atom-containing compounds in future studies.”
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

































































